
��֪��BaSO4(s) + 4C(s) 4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����
4CO(g) + BaS(s)��ҵ�����ؾ�ʯ����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��Ϊԭ�ϣ�ͨ���������������Ȼ������壨BaCl2��nH2O����
�ش��������⣺
��1��������A�Ļ�ѧʽ��_________������ʵ���ҽ��б���ʱ��������������Ĵ���������
a.��NaOH��Һ���� b.��Ũ�������� c.��ȼ
��2���õ�λ�����Һ����������������������ʾ��Ũ�Ƚ�����-���Ũ�ȣ�������g/L��ʾ,����38%��Ũ�������ƺ�����109.5g/L��ϡ����500mL,����Ҫ�IJ����������˲��������� ��
��3��������Ӧ�����ӵ��Լ�R�����������Լ��е�
a.NaOH��Һ b.BaO���� c.��ˮ d.��ʯ��
֤�������Ѿ���ȫ�ķ�����________________________________________________________��
��4�����һ��ʵ��ȷ����Ʒ�Ȼ������壨BaCl2��nH2O���е�nֵ����������ʵ�鲽�裺
�ٳ�����Ʒ��_______ ������_________�����������ƣ�����ȴ �ܳ��� �ݺ��ز�����
���ز�����ָ____________________________________________ _��
�ڢ۲���Ʒ֮���Է��ڸ������н���ʵ���ԭ���� ��
��5�����ؾ�ʯ����̼�Լ��Ȼ��ƹ�ͬ���գ�����ֱ�ӵõ��Ȼ������÷�Ӧ�Ļ�ѧ����Ϊ
BaSO4+ 4C+CaCl2 4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��
4CO + CaS+ BaCl2�������������дӱ��պ�Ĺ����з���õ��Ȼ��������ʵ�����̵���ƣ���֪�Ʋ�����ˮ�����������ᣩ��
����������д�������ƣ�
��1��SiO2 c
��2���ձ� 500mL����ƿ ��ͷ�ι�
��3��b��ȡ�ϲ���Һ��С�Թ��У���������������Һ��������������˵��������ȫ��
��4�����ȡ����������ٽ��м��ȡ���ȴ��������ֱ���������γ����Ľ��������0.001gΪֹ����ֹ��ȴ���������տ����е�ˮ���������ʵ����
��5��
���������������1����ҵ�����ؾ�ʯ�����Ҫ�ɷ�BaSO4������ΪFe2O3��SiO2��������ݵ���SiO2��������֪�������Ļ�ѧ����ʽ��֪������������ΪCO������CO�ķ����ǽ�CO��ȼ��
��2��������Һ���貣�������У��ձ���500mL����ƿ����ͷ�ιܡ���������
��3��BaO��ˮ��Ӧ���������������ܹ��������ӳ������Ҳ������µ����ʣ���ѡ�õ��Լ�ΪBaO���壻֤�������Ѿ���ȫ�ķ�����ȡ�ϲ���Һ��С�Թ��У���������������Һ��������������˵��������ȫ��
��4����ʵ���ԭ�������ü��Ⱥ������ǰ�����������Ȼ��������е�ˮ�����ʵ�����ʵ��������¢ٳ�����Ʒ�ڼ��Ȣ۸���������ȴ �ܳ��� �ݺ��ز��������ز�����ֱָ���������γ����Ľ��������0.001gΪֹ���ڢ۲���Ʒ֮���Է��ڸ������н���ʵ���ԭ����Ϊ�˷�ֹ��ȴ���������տ����е�ˮ���������ʵ����
��5������BaSO4+ 4C+CaCl2 4CO + CaS+ BaCl2��֪��Ӧ��һ����̼�ݳ���Ҫ��ӱ��պ�Ĺ����з���õ��Ȼ���������Ҫ�ӻ����Һ�з���CaS����CaS����ˮ����ֱ�ӹ��˼��ɵõ�BaCl2��Һ��ʵ��������£�
4CO + CaS+ BaCl2��֪��Ӧ��һ����̼�ݳ���Ҫ��ӱ��պ�Ĺ����з���õ��Ȼ���������Ҫ�ӻ����Һ�з���CaS����CaS����ˮ����ֱ�ӹ��˼��ɵõ�BaCl2��Һ��ʵ��������£�
���㣺���ƶϡ�ʵ��������ʹ�á�ʵ���Լ���ѡ��ʵ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CoCl2��6H2O��һ������Ӫ��ǿ������һ������ˮ�ܿ�(��Ҫ�ɷ�ΪCo2O3��Co(OH)3����������Fe2O3��Al2O3��MnO��)��ȡCoCl2��6H2O�Ĺ����������£�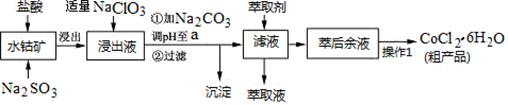
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���(��������Ũ��Ϊ��0.01mol/L)
| ������ | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����(LiCoO2)����ӵ����һ��Ӧ�ù㷺�����͵�Դ��ʵ���ҳ������÷Ͼ����������ӵ�ػ�����������ͭ���ܡ��Ԫ�أ�ʵ��������£�
(1)����ݹ����У������ܽ�����ӷ���ʽΪ__________________________
(2)��ҺA�м���������Һ��ʹCoԪ����CoC2O4��2H2O������ʽ���������������Ʊ������ܼ��ܷ۵���Ҫԭ�ϡ��ڿ�����CoC2O4��2H2O���ȷֽ�ʧ�����ݼ��±����벹���������е��ȷֽⷽ��ʽ��
| ��� | �¶ȷ�Χ/�� | �ȷֽⷽ��ʽ | ����ʧ���� |
| �� | 120��220 | | 19.67% |
| �� | 280��310 | | 56.10% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ӵĹ�ҵ��ˮ����������ͼ��ͼ��ʾ��
��1������������豸���н��е���_________��������д�������ƣ���ʵ��������һ��
����������____________����д�������ƣ����С�
��2�����豸������豸�������A��_____ ____�����豸������豸��������B��______________��������д���ʵĻ�ѧʽ��
��3�����豸���з�����Ӧ�Ļ�ѧ����ʽΪ��_______ ________��
��4�����豸���У�����B��ˮ��Һ��CaO��Ӧ������NaOH��H2O��________��ͨ��________��������д�������ƣ�������ʹ��������롣
��5��ͼ�У���ѭ��ʹ�õ�������C6H6��CaO��________��__________��������д���ʵĻ�ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ƣ�NaClO2����Ҫ�����ġ���ֽҵ��Ư����Ҳ����ʳƷ������ˮ�����ȣ��������������ֽ⡣�������Ƶ�Ϊԭ���Ʊ��������ƵĹ����������£�
��1����ߡ���Ӧl����Ӧ���ʵĴ�ʩ��________________________��д��һ�����ɣ���
��2������Ӧ2������������_____________���÷�Ӧ�Ļ�ѧ����ʽΪ__________________��
��3����ȡ����ѹ�����������á���ѹ��������ԭ����__________________��
��4���ӡ�ĸҺ���пɻ��յ���Ҫ������__________________________��
��5������ȴ�ᾧ����______________����������ƣ����ɻ�ôֲ�Ʒ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�������������NaI��KCl��Na2CO3��Na2SO4��CaCl2��Cu(NO3)2�е�һ�ֻ�����ɣ�Ϊ�˼������������ʣ���������ʵ�飺
��ȡ������������ˮ���õ���ɫ����Һ��
��������Һ�еμ��Ȼ�����Һ���а�ɫ�������ɣ�
�۹��ˣ��������м���������ϡ���ᣬ���ֳ���û��ȫ���ܽ�������ɫ��ζ���������ɡ�
������Һ�м������������Ƶ���ˮ���ټ����������ͣ������ã��ϲ�Һ����Ϻ�ɫ��
��1�����жϣ����������п϶����� ��
һ��û�� ��
���ܺ��� ��
��2���Կ��ܺ��е����ʣ���ν���ʵ���Խ�һ�����顣
��
��3��ʵ����з����Ļ�ѧ��Ӧ���� ��Ӧ���Ӧ���ͣ�����Ҫʵ��������ƽ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̼��﮹㷺Ӧ�����մɺ�ҽҩ������,�Ԧ¡�﮻�ʯ(��Ҫ�ɷ�ΪLi2O��Al2O3��4SiO2)Ϊԭ���Ʊ�Li2CO3�Ĺ�����������:
��֪:Fe3+��Al3+��Fe2+��Mg2+������������ʽ��ȫ����ʱ,��Һ��pH�ֱ�Ϊ3.2��5.2��9.7��12.4;Li2SO4��LiOH��Li2CO3��303 K�µ��ܽ�ȷֱ�Ϊ34.2 g��12.7 g��1.3 g��
��1�������ǰ,�¡�﮻�ʯҪ�����ϸ������Ŀ������������������������������������������
��2���������,�����õ���������Һ�к���Li+��SO42-��������Al3+��Fe3+��Fe2+��Mg2+��Ca2+��Na+������,���ڽ����¼���������(�ʯ��ʯ�������Ȼ��ơ���ϡ���ᡱ)�Ե�����Һ��pH��6.0~6.5,����������������,Ȼ�����õ�����Һ��
��3���������,��������H2O2��Һ��ʯ�����Na2CO3��Һ���μ������Һ��,�ɳ�ȥ�����ʽ�������������������������������������������
��4���������,���ɳ��������ӷ���ʽΪ�� ��
��5����ĸҺ�пɻ��յ���Ҫ������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ྦྷ�裨�赥�ʵ�һ�֣�����Ϊ�����Ӵ��õĻ�ʯ�����Ʊ��и�������SiCl4Ϊ������������Ⱦ�ܴ�����ˮǿ��ˮ�⣬�ų��������ȡ��о���Ա����SiCl4ˮ�����ɵ�����ͱ���ۣ���Ҫ�ɷ�ΪBaCO3���Һ�������þ�����ӣ����Ʊ�BaCl2 ? 2H2O�������������£�
��֪�� �� ������Fe3+��Mg2+ ��ȫ������pH�ֱ���3.4��12.4��
�� BaCO3����Է���������197�� BaCl2 ? 2H2O����Է���������244��
��1��SiCl4����ˮ�ⷴӦ�Ļ�ѧ����ʽ__________________________________
��2�������£�SiCl4 (g) ��H2��ԭ����ȡ���ȺܸߵĹ裬����Ӧ����1mol����ת��ʱ����
59 kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪ__________________________________
��3���ӱ���۵���pH=7�������ǣ�
��ʹBaCO3ת��ΪBaCl2 ��_______________________________
��4����������A�����ӷ���ʽ________________________________________
��5��BaCl2��Һ��__________��_________�����ˡ�ϴ�ӣ��پ���ո����õ�BaCl2 ? 2H2O
��6��10�ֺ�78.8% BaCO3�ı�������������������BaCl2 ? 2H2O___________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ȥ�����ڵ����ʣ�д�������Լ��Ļ�ѧʽ
��1��Cl��(SO42��)�� ��2��SO42��(CO32��)�� ��3��Fe2��(Cu2��)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com