
����˵����ȷ����
A���к͵�����������ʵ���Ũ������ʹ�����Һ����������NaOH��Һ���ڴ���
B�������£�20 LpH=12��Na2CO3��Һ�к��е�OH��������Ϊ0.2NA
C����0.1 mol/LCH3COOH��Һ�м�������CH3COONa�� �壬��Һ�� ����
����
D��һ���¶��£�10mL 0.50mol��L��1 NH4Cl��Һ��20mL 0.25mol��L��1 NH4C1��Һ��NH4+���ʵ�����ͬ
B
��������
���������A����c•V��֪n(HCl)=n(CH3COOH)�����к� NaOH������ͬ������B�������£�c(OH��)=Kw/10��pH=0.01mol/L����c•V��֪n(OH��)=0.2mol����n•NA��֪��N(OH��)=0.2NA����ȷ��C������c(CH3COO��)��CH3COOH CH3COO��+H+�ĵ���ƽ�����ƣ�c(H+)��С��n(CH3COOH)������
CH3COO��+H+�ĵ���ƽ�����ƣ�c(H+)��С��n(CH3COOH)������ ��С��������ƽ�ⳣ��Ka=
��С��������ƽ�ⳣ��Ka= ���䣬����D����c•V��֪n(NH4Cl)��Ϊ0.005mol������NH4Cl���Σ�NH4+�ܲ���ˮ�⣬��ԽϡԽˮ�⣬��ǰ������n(NH4+)��С�ں��ߣ�����
���䣬����D����c•V��֪n(NH4Cl)��Ϊ0.005mol������NH4Cl���Σ�NH4+�ܲ���ˮ�⣬��ԽϡԽˮ�⣬��ǰ������n(NH4+)��С�ں��ߣ�����
���㣺����������Һ������ƽ�⡢��ҺpH������ˮ�⡢���ʵ���Ũ�ȡ����ʵ��������֪ʶ��


 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1mol CH3OCH3��1mol H2O | 2mol CH3OH | 1mol CH3OH |
| CH3OH��Ũ�ȣ�mol/L�� | c1 | c2 | c3 |
| ��Ӧ�������仯 | ����a kJ | �ų�b kJ | �ų�c kJ |
| ƽ��ʱ�����L�� | V1 | V2 | V3 |
| ��Ӧ��ת���� | �� 1 | �� 2 | �� 3 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 �ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�
�ǽ���Ԫ�ص��ж����������NO��NO2��N2O4�ȣ�| ʱ�䣨s�� | 0 | 20 | 40 | 60 | 80 |
| n��NO2��/mol | 0.40 | n1 | 0.26 | n3 | n4 |
| n��N2O4��/mol | 0.00 | 0.05 | n2 | 0.08 | 0.08 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
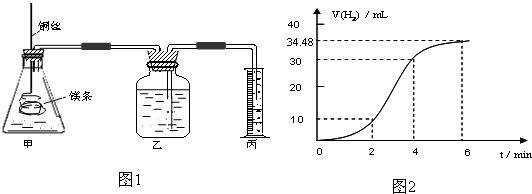
| ʵ���� | �� | �� | �� | �� |
| 10%H2O2�����/mL | 5.0 | 5.0 | V1 | V2 |
| 20%��������/mL | 0 | 0.5 | 1.0 | V3 |
| ˮ�����/mL | 15 | 14.5 | V4 | 13.5 |
| ����ʱ��t/s | t1 | t2 | t3 | t4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com