
| 46��52.2% |
| 12 |
| 46��13% |
| 1 |
| 46-12��2-6 |
| 16 |
| 46��52.2% |
| 12 |
| 46��13% |
| 1 |
| 46-12��2-6 |
| 16 |
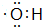 ���ʴ�Ϊ��
���ʴ�Ϊ��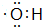 ��
��| Cu |
| �� |
| Cu |
| �� |
| Ũ���� |
| �� |
| 132g |
| 88g |
| 69g |
| 184g |
| Ũ���� |
| �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����50% | B��=50% |
| C����50% | D�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
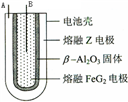 X��Y��Z��M��G����Ԫ�ط������������ڣ�ԭ��������������X��Zͬ���壬���γ����ӻ�����ZX��Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ���ش��������⣺
X��Y��Z��M��G����Ԫ�ط������������ڣ�ԭ��������������X��Zͬ���壬���γ����ӻ�����ZX��Y��Mͬ���壬���γ�MY2��MY3���ַ��ӣ���ش��������⣺| ��� |
| �ŵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ܢݢ� | B���ڢܢݢ� |
| C���ڢܢ� | D���ڢۢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
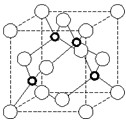 �� X��Y��Z��W����Ԫ�أ�����X�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29���ش��������⣺
�� X��Y��Z��W����Ԫ�أ�����X�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
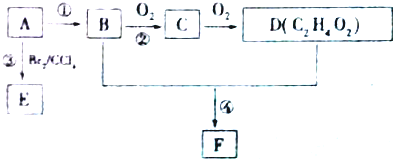
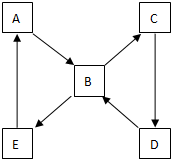 ��ͼ��ʾij��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ��������A����ij��̬���ʻ������ɻ�����B��
��ͼ��ʾij��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ��������A����ij��̬���ʻ������ɻ�����B���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
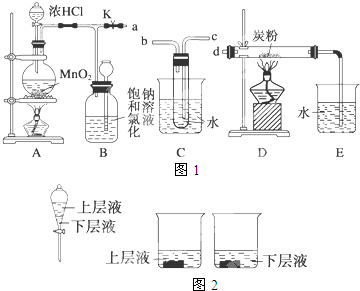
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com