
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�
K+��NH4+��Cl����Mg2+��Ba2+��CO32����SO42������ȡ������Һ��100 mL��������ʵ�飺�ٵ�һ�ݼ���AgNO3��Һ�г����������ڵڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�����������0.04 mol���۵����ݼ�����BaCl2��Һ�ø������6.27 g������������ϴ�ӡ������������Ϊ2.33 g����������ʵ�飬�ش��������⣺(1)һ�������ڵ�������________������ȷ���Ƿ���ڵ�������________��
(2)��ȷ����Һ�п϶����ڵ������Ӽ���Ũ��(�ɲ�����)��
���ӷ���________��Ũ��________��
���ӷ���________��Ũ��________��
���ӷ���________��Ũ��________��
(3)K+________(�һ������һ������)���ڣ�������________��
�����𰸣�
(1)Mg2+��Ba2+��Cl������(2)CO32����0.2 mol/L��SO42����0.1 mol/L
����
(3)һ��������ʵ�������жϣ���Һ�п϶����ڵ�������NH4+��CO32����SO42����NH4+�����ʵ���Ϊ0.04 mol��CO32����SO42�������ʵ����ֱ�Ϊ0.02 mol��0.01 mol�����ݵ���غ㣬K+һ������������ʾ���ɵڶ���ʵ���֪����
NH4+��������ʵ���г������ɣ��ҳ�������ȫ��ʧ��˵������CO32����SO42����ͬʱ�ó�һ��������Mg2+��Ba2+��������ʹ���������������ӽ϶࣬����ȷ��Cl���Ƿ�һ�����ڣ�


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
+ 4 |
2- 3 |
2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ѧϰ�ܱ�����ѧ���˽̿α��һ��(����1)��2009��2010ѧ�ꡡ��8�ڡ��ܵ�164�� �˽̿α�� ���ͣ�058
��֪NH4+��OH��![]() NH3����H2O������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��Cl����NH4+��Mg2+��Ba2+��CO32����SO42������ȡ����200 mL��Һ��������ʵ�飺�ٵ�һ���м���AgNO3��Һ�г����������ڵڶ����м�������NaOH��Һ���Ⱥ��ռ�������0.05 mol���۵������м�������BaCl2��Һ�ø������6.27 g������������ϴ�ӡ������������Ϊ2.33 g��
NH3����H2O������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��Cl����NH4+��Mg2+��Ba2+��CO32����SO42������ȡ����200 mL��Һ��������ʵ�飺�ٵ�һ���м���AgNO3��Һ�г����������ڵڶ����м�������NaOH��Һ���Ⱥ��ռ�������0.05 mol���۵������м�������BaCl2��Һ�ø������6.27 g������������ϴ�ӡ������������Ϊ2.33 g��
(1)ԭ��Һ��һ�����ڵ�������________��
(2)ԭ��Һ��һ�������ڵ�������________��
(3)��д��ԭ��Һ��һ�����ڵ������ӵ����ʵ���Ũ�ȣ�________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�����ڶ���������⻯ѧ�Ծ��������棩 ���ͣ������
��11�֣���ͼ����ĸ�����������ʾ�Ϊ��ѧ��ѧ�������ʡ�����A���ճ������в���ȱ�ٵ����ʣ�Ҳ�ǻ��������ϵ���Ҫԭ�ϣ�������C��D��HΪ���嵥�ʡ�����E��M��NΪ������N�ǵؿ��к������Ľ���Ԫ�ء�Y�Ǻ��ɫ��������Щ������һ�������´�������ת�� ��ϵ��������Щ��Ӧ����������Ѿ���ȥ���Իش��������⣺
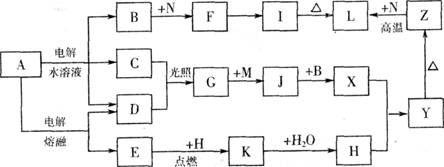
(1) ��ҵ�ϣ��ڵ��A��Һ���豸�н����������������� �����������豸���ƣ�
(2) д��A��ˮ��Һ�������ӷ���ʽ ��
(3) д��K��CO2��Ӧ�Ļ�ѧ����ʽ ��
(4) Y��NaClO��B�Ļ����Һ���ã����Ʊ���ɫˮ������(Na2MO4)��һ�ַ�����д���÷�Ӧ�����ӷ���ʽ ��
(5) һ������Z��N�Ļ�����Ϊ���ȷ֣�һ��ֱ������������������Һ��������Ϊamol����һ�ݸ����³�ַ�Ӧ�������ǹ����������ķ�Ӧ����Ĺ������������������������Ϊbmol������a:b=9:7����������Z��N�����ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����Al3����Fe3����Mg2����Ba2����NH4����Cl����CO32����SO42������ȡ����100mL��Һ��������ʵ�飺
�ŵ�һ�ݼ��뼸��AgNO3��Һ���г���������
�Ƶڶ��ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.03mol���������ɣ�ͬʱ�õ���Һ��
���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g
�ȵ����ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65g��
����˵������ȷ����
A������Ǻ����ȷ��CO32��һ�������� B�����Բ���Ŷ�ʵ����۲���Ӱ��
C������ȷ��ԭ��Һ�Ƿ���K����Cl��
D�����Ѳ���Ʋ���������ͨ�벽��ŵ���Һ�У��ֿɲ���0.78g����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com