
(1)�밴Ҫ����д��ѧ����ʽ��
������ͭ���뷽��ʽΪ
________________________________________________________________________��
������ͭˮ������ӷ���ʽΪ
________________________________________________________________________��
������ͭ��Һ������������Ӧ�����ӷ���ʽΪ____
____________________________________________________________________
________________________________________________________________________��
(2)����NaHSO4��Һ�У���μ���Ba(OH)2��Һ�����ԣ�������Ӧ�����ӷ���ʽΪ____
____________________________________________________________________
________________________________________________________________________��
��������Һ�У������μ�Ba(OH)2��Һ���˲���Ӧ�����ӷ���ʽΪ____
____________________________________________________________________��
����Ba(OH)2��Һ�У���μ���������Һ��Ba2��ǡ����ȫ�������䷴Ӧ�����ӷ���ʽΪ
________________________________________________________________________
________________________________________________________________________��
��������Һ�У������μ�������Һ���˲���Ӧ�����ӷ���ʽΪ___
_____________________________________________________________________��
(3)����������ӷ���ʽ��
��KOH��Һ������Ca(HCO3)2��Һ��Ӧ
________________________________________________________________________��
��KOH��Һ������Ca(HCO3)2��Һ��Ӧ
________________________________________________________________________��
��Ca(OH)2��Һ������NaHCO3��Һ��Ӧ
________________________________________________________________________��
������Ca(OH)2��Һ��NaHCO3��Һ��Ӧ
________________________________________________________________________��
������(1)Ӧ��ע���������෴Ӧ����ʽ��д���ϵIJ�ͬ��(2)Ba(OH)2��NaHSO4�ķ�Ӧ�����⣬Ӧ���ע���ж��ڷ�Ӧ���������ʹ�����Ba(OH)2�������ķ�Ӧ����Ba2����SO![]() ��OH����Al3����������Ӧ�����жϷ�Ӧ��������ʱӦͬʱ���ǡ�(3)����Ca(HCO3)2�������ģ�KOH������Խ��٣�OH����HCO
��OH����Al3����������Ӧ�����жϷ�Ӧ��������ʱӦͬʱ���ǡ�(3)����Ca(HCO3)2�������ģ�KOH������Խ��٣�OH����HCO![]() ��Ӧ����������H2O��CO
��Ӧ����������H2O��CO![]() ����ʣ��HCO
����ʣ��HCO![]() δ��Ӧ��
δ��Ӧ��
�𰸡�(1)��CuSO4===Cu2����SO![]()
��Cu2����H2OCu(OH)2��2H��
��Ba2����SO![]() ��2OH����Cu2��===BaSO4����Cu(OH)2��
��2OH����Cu2��===BaSO4����Cu(OH)2��
(2)��2H����SO![]() ��2OH����Ba2��===BaSO4����2H2O
��2OH����Ba2��===BaSO4����2H2O
Ba2����SO![]() ===BaSO4��
===BaSO4��
��Al3����2SO![]() ��4OH����2Ba2��===2BaSO4����AlO
��4OH����2Ba2��===2BaSO4����AlO![]() ��2H2O��Al3����3AlO
��2H2O��Al3����3AlO![]() ��6H2O===4Al(OH)3��
��6H2O===4Al(OH)3��
(3)��OH����Ca2����HCO![]() ===CaCO3����H2O
===CaCO3����H2O
��2OH����Ca2����2HCO![]() ===CaCO3����2H2O��CO
===CaCO3����2H2O��CO![]()
��Ca2����2OH����2HCO![]() ===CaCO3����2H2O��CO
===CaCO3����2H2O��CO![]()
��HCO![]() ��Ca2����OH��===CaCO3����H2O
��Ca2����OH��===CaCO3����H2O



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2010?�Ĵ����ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺
��2010?�Ĵ����ⱻ��Ϊ������Ԫ�ء�����ѧ�����ز����ɷ�ֹ��ȱ����������أ�KIO3���ǹ��ҹ涨��ʳ�μӵ�������ľ���Ϊ��ɫ��������ˮ������������Խ���������������⻯�����þ����ɵ��ʵ⣮�Ե�Ϊԭ�ϣ�ͨ������Ʊ�����ص�ʵ��װ����ͼ��ʾ����ش��������⣺| ʵ�鷽�� | ʵ�������� |
ȡ�������������Һ���Թ��У���ϡ�����ữ����뼸�ε�����Һ���۲��Ƿ������ ȡ�������������Һ���Թ��У���ϡ�����ữ����뼸�ε�����Һ���۲��Ƿ������ |
�����������˵����I-�������������˵����I-�� �����������˵����I-�������������˵����I-�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
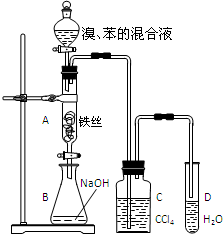 ij��ѧ����С������ͼװ����ȡ�屽��̽���÷�Ӧ�����ͣ������Һ©���м��뱽��Һ�壬�ٽ����Һ���뷴Ӧ��A��A�¶˻����رգ��У�
ij��ѧ����С������ͼװ����ȡ�屽��̽���÷�Ӧ�����ͣ������Һ©���м��뱽��Һ�壬�ٽ����Һ���뷴Ӧ��A��A�¶˻����رգ��У�| Fe |
| Fe |
| ���Թ�D�м�����Լ� | ��֤������Һ�巢��ȡ����Ӧ������ | |
| ����һ | ||
| ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| NaOH��Һ |
| �ữ |
| NaOH��Һ |
| �ữ |
| NaOH��Һ |
| �ữ |


| ʵ�鲽�� | �����Լ� | �� �� | �� �� |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 |


 Ϊʵ���������ʵ�ת��
Ϊʵ���������ʵ�ת��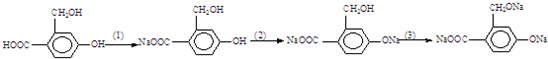
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�058

��1���ܽ���Ʒ���õ�������________��
��2������Ʒ��Һ�м����A��Һ��________��
AҪ�ữ��Ŀ����________�����������ữ��ԭ����________��AҪ������ԭ����________��
��3��ͨ��������ɫ��Һa���ж�A�Ƿ������ȡ����a��Һ���μ�________���������A�ѹ�����
��4����ɫ����b�ں��ǰ��Ӧ�ڹ�������������ˮ���ϴ�ӣ���ԭ����________��Ϊ�˼�������Ƿ�ϴ����Ӧ�����ϴ��Һ�еμ�________����________�����������ϴ����
��5��ͨ������ʵ��ⶨ������Ʒ���ȵ���ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��һ���������������Լ��������������ʣ�Ϊ�˲ⶨ�Լ��Ĵ��ȣ�������ͼ�������ķ�������ʵ�飬�밴Ҫ����д���¸���հס�

��1���ܽ���Ʒ���õ�������________��
��2������Ʒ��Һ�м����A��Һ��________��
AҪ�ữ��Ŀ����________�����������ữ��ԭ����________��AҪ������ԭ����________��
��3��ͨ��������ɫ��Һa���ж�A�Ƿ������ȡ����a��Һ���μ�________���������A�ѹ�����
��4����ɫ����b�ں��ǰ��Ӧ�ڹ�������������ˮ���ϴ�ӣ���ԭ����________��Ϊ�˼�������Ƿ�ϴ����Ӧ�����ϴ��Һ�еμ�________����________�����������ϴ����
��5��ͨ������ʵ��ⶨ������Ʒ���ȵ���ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com