
��������ʮ�����ʣ�
��H2����������CaO����CO2����H2SO4����Ba(OH)2���ߺ��ɫ����������Һ�壻�ఱˮ����ϡ�����Al2(SO4)3
���������ʰ����ʵķ������д����Ŀհ״��������ʱ�ţ���
| ����� | �������� | ������ | ��Һ | ���� | ����� |
| ���ڸ�������� | | | | | |
��1�� �������� ������ ��Һ ���� ����� ���ڸ�������� �� �ۢ� ��� �� �ۢݢޢ�
��2��Ba(OH)2 + 2HNO3 = Ba(NO3)2 + 2H2O
��3��Al2(SO4)3 = 2Al3��+ 3SO42��
��4��Ba2��+2OH��+CO2 = BaCO3��+ H2O
��5��HNO3 1�U1 0��6 mol Al + 4H��+NO3�� =Al3��+NO��+ 2 H2O
���������������1��������ֻ�н�����Ԫ����ɵĽ������ʣ���Na2O�͢�CO2�к�������Ԫ������һ��Ԫ������Ԫ�أ�����������ఱˮ�к���NH3��H2O��NH3��H2O���������ǻ�����ϡ�������H+��NO3-��H2O�����������ڻ�����������ֱ��С��1nm,����������Һ���ߺ��ɫ����������Һ������Fe(OH)3��ֱ����1��100nm֮�䣬���ڽ��塣�ۢݢޢ���ˮ��Һ��������״̬���ܵ��磬�����Ļ��������ڵ���ʣ��ʴ�Ϊ������� �������� ������ ��Һ ���� ����� ���ڸ�������� �� �ۢ� ��� �� �ۢݢޢ�
��2������ʮ������������������֮��ɷ������ӷ�Ӧ��H++OH-�TH2O������������Ϊ�����Ե�ǿ����ǿ�ᷴӦ���ɿ����Ե��εķ�Ӧ������������µķ�Ӧ����ʽΪ��Ba��OH��2+2HNO3�TBa��NO3��2+2H2O��
��3��Al2(SO4)3 = 2Al3��+ 3SO42��
��4�������Ķ�����̼���Ժ�ǿ������������Ӧ����̼�ᱵ��ˮ�������ӷ���ʽΪ��Ba2++2OH-+CO2=BaCO3��+H2O��
��5����֪Al+4HNO3=Al��NO3��3+NO��+2H2O��HNO3��NO����Ԫ�ػ��ϼ۴�+5����Ϊ+2��HNO3����������1molAl��0������Ϊ+3�ۣ�ʧȥ3mol���ӣ�
1molHNO3���ϼ۴�+5����Ϊ+2�õ�3mol���ӣ����Ի�ԭ���������������ʵ�����ȣ�n��Al��=n/M=5��4g/27g/mol=0��2mol������ת�Ƶ��ӵ����ʵ���Ϊ0��2mol��3=0��6mol��
Al��4HNO3��Ӧ�����ӷ���ʽΪAl+4H++NO3-=Al3++NO��+2H2O��
���㣺�������ʵķ��ࡢ���ʵ����ļ����Լ����ʵ����ʷ���֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����ڸ�ѹ�¸����������Ⱥ�����ȴ���ɵøֻ�ɫ���塪�����ף���ת���������£�����  ���ף����ױȰ����ȶ����ṹ��ʯī���ơ�����������ȷ����
���ף����ױȰ����ȶ����ṹ��ʯī���ơ�����������ȷ����
| A������ת��Ϊ���������ȷ�Ӧ |
| B�����������Ϊͬ���칹�� |
| C������ת��Ϊ������������ԭ��Ӧ |
| D�������ܵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������һ����Ҫ�Ļ���ԭ�ϣ������Ʊ�һϵ�����ʡ�����˵���������( )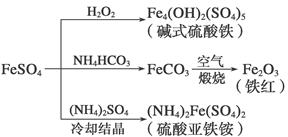
| A����ʽ������ˮ���ܲ���Fe(OH)3���壬��������ˮ�� |
| B��Ϊ��ֹNH4HCO3�ֽ⣬����FeCO3���ڽϵ��¶��½��� |
| C������KSCN��Һ����(NH4)2Fe(SO4)2�Ƿ����� |
| D�������£�(NH4)2Fe(SO4)2��ˮ�е��ܽ�ȱ�FeSO4�Ĵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ʾ��ѧ֪ʶ������ij�ֹ��ԶԻ�ѧ���ʽ��з��࣬��
�����ǵ�Ч�������磬����̼�ظֵĺ�̼���������Ϊ��̼�֡���̼�֡���̼�֣�����������෨�ɽ����ʾΪ��
������һ�������ش�
(1)25�潫pH����Һ����ԵĹ�ϵ���Եر�ʾ�����������ϣ�
(2)ij��ѧ��ȤС�����о�H2SO4��KCl��NaCl��Na2CO3��Na2SO3��NaOH�������ʵ����ʣ���������о�����������������о�������
���������ǰ����ᡢ��η��࣬Ȼ��ֱ�����ˮ�õ���Һ������ʵ�顣
���������ǰ������Ρ����κ�������������࣬Ȼ��ֱ�����ˮ�õ���Һ������ʵ�顣
�ٸ��ݷ�������з���ʱ����ʵ����KCl��NaCl��Һ��pH����7��H2SO4��Һ��pHС��7��Na2SO3��Na2CO3��NaOH��Һ��pH����7���ɴ��е�ͬѧ�����෨˼���Na2SO3��Na2CO3��NaOH������Ϊ�����Ƿ������Ϊʲô�� ��
���ڷ������У�ijͬѧ�������������е�KCl��NaCl�����������ʻ��ʱ������ͬ�������֣������һ�ּķ����������������� ��
���ڷ������У��������������������е� �ɼ������֣��йط�Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ ��
�ܸ���ȤС���е�һλͬѧ��Ϊ�������Ը����Ƿ�����Ԫ�ؽ������������ʷ�ΪNa2SO3��Na2CO3��NaCl��NaOH��H2SO4��KCl���ࡣ����H2SO4�������ֺ���Ԫ�ص�����ʱ��Na2SO3��Na2CO3�����õ������֣�������NaCl��NaOHʱȴ�����������������һ��ʵ���������һ���⣺ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������ʹ�õĽ���֮һ��������ѧ֪ʶ���ش��������⡣
��1������Fe(OH)3�����FeCl3��Һ�ķ����� ��
��2�����ӹ�ҵ��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ������ӡˢ��·�壬��д��FeCl3��Һ��ͭ��Ӧ�����ӷ���ʽ�� ��
��3��ij�о���ѧϰС��Ϊ�ⶨFeCl3��ʴͭ��������Һ����ɣ�����������ʵ�飺
��ȡ����������Һ������KSCN��Һ�ʺ�ɫ�������Һ�к��еĽ����������� ������Һ��ɵIJⶨ��ȡ50.0mL������Һ������������AgNO3��Һ����21.525g��ɫ����������Һ��c(Cl��)�� mol��L��1��
����֤����Һ�к���Fe2+����ȷ��ʵ�鷽���� ��
A���۲���Һ�Ƿ��dz��ɫ
B��ȡ������Һ���������Ը��������Һ������ɫ��֤������Fe2+
C��ȡ������Һ��������ˮ���ٵ���KSCN��Һ������Ѫ��ɫ��֤��ԭ��Һ�к���Fe2+
��4������ʦ��������ӡˢ��·��ķ�ˮ�л���ͭ�������FeCl3��Һ��������·�����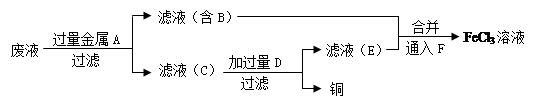
������C�Ļ�ѧʽΪ ��
�ڼӹ���D������Ӧ�����ӷ���ʽΪ ��
��ͨ��F������Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1������˵������ȷ����________(����ĸ���)��
| A��60��������ı���HQE�����������ȫ���������������������ڼ��������� |
| B�����ȵĴ�����Һϴ��մ�����۵�����ʱ��������Ҫ�ǻ�ѧ�仯 |
| C��Ӣ�������ѧ�Ҹ�����ڡ�������ά�еĴ���Ӧ���ڹ�ѧͨ�ŷ��桱������ͻ���Գɾͣ��������2009��ŵ��������ѧ����������Ʒ�Ļ���ԭ��ΪSiO2 |
| D��Һ����Һ�ȡ�Һ̬�Ȼ��ⶼ�Ƿǵ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ�����ﳣ�õĸ�����У�����ʯ�ң��ڹ����������ƣ��۱�ɫ�轺����Ҫ�ɷ��Ƕ������裬�����в�����������ˮ�Ȼ���(CoCl2)��ָʾ���ݣ������������ף�����ˮ�Ȼ��ƣ���Ũ���ᣬ��ʯ��(��Ҫ�ɷ����������ơ�������)�ȡ�
��1�����������У����ڴ��������__________��
A���٢ڢ� B���ڢܢ� C���٢ڢܢ� D��ȫ��
��2�������ڡ��ܡ��ݡ������ָ�����У�����Ҫ��ѧ�ɷ��������������Ϊ______��________��________��______��������ţ�
A���� B���� C���� D��������
��3���轺����ˮ�Ȼ���(CoCl2)����ɫ����ˮ���Ϊ�ۺ�ɫ��CoCl2?6H2O���ñ仯��������____________(������仯����ѧ�仯��)��
��4�����������У����ù����������Ƹ�����ǣ� ��
A��CO2 B��HCl C��NO2 D��NH3 E��NO
��5����ʯ�ҳ�����ʳƷ����������ú���ʧȥ������������ԭ��Ϊ_________________ (�û�ѧ����ʽ��ʾ)
��6������������У�����Ũ����ΪҺ�����������й���Ũ�����������ȷ���ǣ� ��
A��Ũ���������ˮ�ԣ������ʹ����̿��
B��Ũ�����ڳ����¿�Ѹ����ͭƬ��Ӧ�ų�������������
C��Ũ����ΪҺ������������Ч�ʸߣ������ڸ������е�����
D��Ũ�����ڳ������ܹ�ʹ�������Ƚ����ۻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(14��)NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
(1)NH4Al(SO4)2������ˮ������������____________________________________________(�ñ�Ҫ�Ļ�ѧ������������˵��)��
(2)��ͬ�����£�0.1 mol��L��1NH4Al(SO4)2��c(NH4+)________(����ڡ��������ڡ���С�ڡ�)0.1 mol��L��1NH4HSO4��c(NH4+))��
(3)��ͼ��0.1 mol��L��1���ֵ������Һ��pH���¶ȱ仯��ͼ��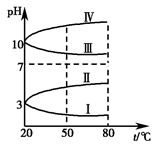
�����з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯��������________(��д���)������pH���¶ȱ仯��ԭ����____________________________��
��20 ��ʱ��0.1 mol��L��1NH4Al(SO4)2��2c(SO42-)��c(NH4+))��3c(Al3��)��________�������㾫ȷֵ��(4)����ʱ����100 mL 0.1 mol��L��1 NH4HSO4��Һ�еμ�0.1 mol��L��1 NaOH��Һ���õ���ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��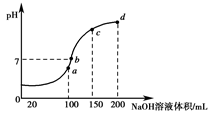
�Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶������________�㣻��b�㣬��Һ�и�����Ũ���ɴ�С������˳����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���з�Ӧ�����ӷ���ʽ�������
| A����ˮ�ӵ��廯����Һ�У�2 Br��+ I2=== 2I�� + Br2 |
| B������ͨ��⻯����Һ�У�2I��+ Cl2=== 2Cl��+ I2 |
| C����������ˮ��Cl2+ H2O === 2H����+ Cl��+ ClO�� |
| D����������Һ�еμӵ⻯����Һ��Ag����+�� I����===�� AgI�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com