
A��B��D��X��Y���ɶ�����Ԫ����ɵĻ��������X��Y�ֱ��dz�����ǿ�ᡢǿ����Ǵ�������ͼת��(�����������ˮ��ȥ)��
(1)��A��B��Ϊ���壬��ˮ��Һ�з�Ӧ��������D������A���γ��������Ҫ���ʡ���D�Ļ�ѧʽ�� ��B������������Ӧ�Ļ�ѧ����ʽ�� ��
(2)��A��B��D������ͬ�Ľ���Ԫ�أ����Ԫ����Ԫ�����ڱ��е�λ���� ����ҵ����ȡ�ý������ʵĻ�ѧ����ʽΪ�� ���ֽ�X����B����Һ�����������ù��̷��������ӷ���ʽΪ�� ��
��3����AΪ��ɫ��ζ�����壬BΪ�Σ���A��B��D�������ʺ�����ͬԪ�أ���A�ĵ���ʽΪ�� ��A��B��Һ��Ӧ����D�����ӷ���ʽΪ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣�A��B��D��X��Y���ɶ�����Ԫ����ɵĻ��������X��Y�ֱ��dz�����ǿ�ᡢǿ����Ǵ�������ͼת��(�����������ˮ��ȥ)��
(1)��A��B��Ϊ���壬��ˮ��Һ�з�Ӧ��������D������A���γ��������Ҫ���ʡ���D�Ļ�ѧʽ�� ��B������������Ӧ�Ļ�ѧ����ʽ�� ��
(2)��A��B��D������ͬ�Ľ���Ԫ�أ����Ԫ����Ԫ�����ڱ��е�λ���� ����ҵ����ȡ�ý������ʵĻ�ѧ����ʽΪ�� ���ֽ�X����B����Һ�����������ù��̷��������ӷ���ʽΪ�� ��
��3����AΪ��ɫ��ζ�����壬BΪ�Σ���A��B��D�������ʺ�����ͬԪ�أ���A�ĵ���ʽΪ�� ��A��B��Һ��Ӧ����D�����ӷ���ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ױڸ��и�����һ��ѹ������ۻ�ѧ���� ���ͣ������
��14�֣�A��B��D��X��Y���ɶ�����Ԫ����ɵĻ��������X��Y�ֱ��dz�����ǿ�ᡢǿ����Ǵ�������ͼת��(�����������ˮ��ȥ)��

(1)��A��B��Ϊ���壬��ˮ��Һ�з�Ӧ��������D������A���γ��������Ҫ���ʡ���D�Ļ�ѧʽ�� ��B������������Ӧ�Ļ�ѧ����ʽ�� ��
(2)��A��B��D������ͬ�Ľ���Ԫ�أ����Ԫ����Ԫ�����ڱ��е�λ���� ����ҵ����ȡ�ý������ʵĻ�ѧ����ʽΪ�� ���ֽ�X����B����Һ�����������ù��̷��������ӷ���ʽΪ�� ��
��3����AΪ��ɫ��ζ�����壬BΪ�Σ���A��B��D�������ʺ�����ͬԪ�أ���A�ĵ���ʽΪ�� ��A��B��Һ��Ӧ����D�����ӷ���ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��D��X��Y���ɶ�����Ԫ����ɵĻ��������X��Y�ֱ��dz�����ǿ�ᡢǿ����Ǵ�������ͼת��(�����������ˮ��ȥ)��
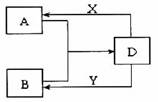
(1)��A��B��Ϊ���壬��ˮ��Һ�з�Ӧ��������D������A���γ��������Ҫ���ʡ���D�Ļ�ѧʽ�� ��B������������Ӧ�Ļ�ѧ����ʽ�� ��
(2)��A��B��D������ͬ�Ľ���Ԫ�أ����Ԫ����Ԫ�����ڱ��е�λ���� ����ҵ����ȡ�ý������ʵĻ�ѧ����ʽΪ�� ���ֽ�X����B����Һ�����������ù��̷��������ӷ���ʽΪ�� ��
��3����AΪ��ɫ��ζ�����壬BΪ�Σ���A��B��D�������ʺ�����ͬԪ�أ���A�ĵ���ʽΪ�� ��A��B��Һ��Ӧ����D�����ӷ���ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[s1]
 |
(1)��A��B��Ϊ���壬��ˮ��Һ�з�Ӧ��������D������A���γ��������Ҫ���ʡ���D�Ļ�ѧʽ�� ��B������������Ӧ�Ļ�ѧ����ʽ�� ��
(2)��A��B��D������ͬ�Ľ���Ԫ�أ����Ԫ����Ԫ�����ڱ��е�λ���� ����ҵ����ȡ�ý������ʵĻ�ѧ����ʽΪ�� ���ֽ�X����B����Һ�����������ù��̷��������ӷ���ʽΪ�� ��
��3����AΪ��ɫ��ζ�����壬BΪ�Σ���A��B��D�������ʺ�����ͬԪ�أ���A�ĵ���ʽΪ�� ��A��B��Һ��Ӧ����D�����ӷ���ʽΪ�� ��
[s1]28��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com