
����6�֣�ʵ������ȡSO2�ķ�Ӧԭ��֮һΪ�� Na2SO3��H2SO4��Ũ��===Na2SO4��SO2����H2O����������װ�����һ��ʵ�飬�ԲⶨSO2ת��ΪSO3��ת���ʣ���![]() ��100�G��
��100�G��
��1����Щװ�õ�����˳���������ҵķ����� �� �� �� �� �� �� �� �� ������ӿڵı�ţ���
��2��ʵ��ʱ���ܵ�������ԭ���� ��
��3�����Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��
��4�������۲쵽�������� ��
��5����n mol Na2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ��Ƶâ�����m g����ʵ����SO2��ת����Ϊ ��
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�ٷ�һ�и�һ6���¿���ѧ�Ծ����������� ���ͣ������
(1)�������С���Ѿ������˻�ѧ��Ӧ�ķ�Ӧ���д�������Ļ�ѧ��Ӧ����ʽ����ƽ����ÿС��2�֣���6�֣�
������������͵�������Ӧ
����ˮ�⣺
CH3CH(OH)CH3�Ĵ�������
(2)��4�֣�.��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��.SO2+2H2O+I2===H2SO4+2HI ��.2HI H2+I2 ��.2H2SO4===2SO2+O2+2H2O
H2+I2 ��.2H2SO4===2SO2+O2+2H2O
��1��һ���¶��£���1L�ܱ������м���1mol HI��g����������Ӧ�����ɵ�I2Ϊ���壬H2���ʵ�����ʱ��ı仯��ͼ��ʾ��0-2 min�ڵ�ƽ����Ӧ���ʦԣ�HI��= 
��2��ʵ������Zn��������ȡH2��Ϊ�˼ӿ췴Ӧ���ʣ����д�ʩ�����е��� ������ţ�
a������Ũ���� b����������CuSO4���� c���ô�п���洿п
d������ e.��п��Ū��п�� f.��98.3%Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡɽ���и�һ2���¿���ѧ�Ծ� ���ͣ�ʵ����
����6�֣�ʵ������ȡSO2�ķ�Ӧԭ��֮һΪ��Na2SO3��H2SO4��Ũ��===Na2SO4��SO2����H2O����������װ�����һ��ʵ�飬�ԲⶨSO2ת��ΪSO3��ת���ʣ��� ��100�G��
��100�G��
��1����Щװ�õ�����˳���������ҵķ����� �� �� �� �� �� �� �� �� ������ӿڵı�ţ���
��2��ʵ��ʱ���ܵ�������ԭ���� ��
��3�����Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��
��4�������۲쵽�������� ��
��5����n mol Na2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ��Ƶâ�����m g����ʵ����SO2��ת����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ��һ6���¿���ѧ�Ծ��������棩 ���ͣ������
(1)�������С���Ѿ������˻�ѧ��Ӧ�ķ�Ӧ���д�������Ļ�ѧ��Ӧ����ʽ����ƽ����ÿС��2�֣���6�֣�
������������͵�������Ӧ
����ˮ�⣺
CH3CH(OH)CH3�Ĵ�������
(2)��4�֣�.��-��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��.SO2+2H2O+I2===H2SO4+2HI
��.2HI H2+I2 ��.2H2SO4===2SO2+O2+2H2O
H2+I2 ��.2H2SO4===2SO2+O2+2H2O
��1��һ���¶��£���1L�ܱ������м���1mol HI��g����������Ӧ�����ɵ�I2Ϊ���壬H2���ʵ�����ʱ��ı仯��ͼ��ʾ�� 0-2 min�ڵ�ƽ����Ӧ���ʦԣ�HI��=

��2��ʵ������Zn��������ȡH2��Ϊ�˼ӿ췴Ӧ���ʣ����д�ʩ�����е��� ������ţ�
a������Ũ���� b����������CuSO4 ���� c���ô�п���洿п
d������ e.��п��Ū��п�� f.��98.3%Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��ɽ��ʡ��һ2���¿���ѧ�Ծ� ���ͣ�ʵ����
����6�֣�ʵ������ȡSO2�ķ�Ӧԭ��֮һΪ�� Na2SO3��H2SO4��Ũ��===Na2SO4��SO2����H2O����������װ�����һ��ʵ�飬�ԲⶨSO2ת��ΪSO3��ת���ʣ���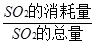 ��100�G��
��100�G��
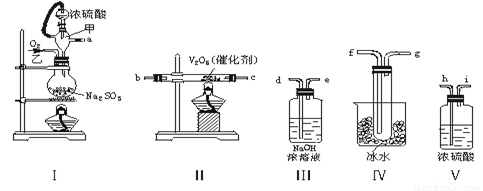
��1����Щװ�õ�����˳���������ҵķ����� �� �� �� �� �� �� �� �� ������ӿڵı�ţ���
��2��ʵ��ʱ���ܵ�������ԭ���� ��
��3�����Ҵ�����ͨ��O2��ΪʹSO2�нϸߵ�ת���ʣ�ʵ��ʱ����Ũ��������ȴ������Ⱥ�˳���� ��
��4�������۲쵽�������� ��
��5����n mol Na2SO3��ĩ������Ũ������д�ʵ�飬����Ӧ����ʱ������ͨ��O2һ��ʱ��Ƶâ�����m g����ʵ����SO2��ת����Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com