
����֪�ⶨ�к��ȵ�ʵ�鲽�����£�����ȡ50mL 0.25 mol/L���ᵹ��С�ձ��У������¶� ����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ� �۽�NaOH��Һ����С�ձ��У���Ͼ��Ⱥ�������Һ�¶ȡ���ش�
��1��NaOH��Һ�Թ�����ԭ�� ��
��2������NaOH��Һ����ȷ������ (����ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������ ��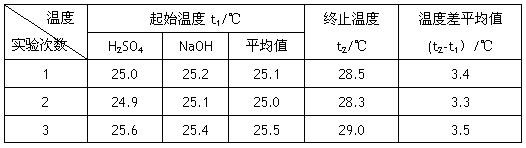
��4������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c="4.18" J��(g����)-1�������ʵ����������к���Ϊ�������� ����д���÷�Ӧ���Ȼ�ѧ����ʽ_________
��5��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų������� ���С�ڡ��������ڡ����ڡ���57.3 kJ��ԭ�������������� ����
��ij�ռ���Ʒ�����������������õ����ʣ�Ϊ�˲ⶨ�䴿�ȣ��������²�����
A����250 mL������ƿ�ж������250 mL�ռ���Һ
B���ü�ʽ�ζ�����ȡ25.00 mL�ռ���Һ����ƿ�У������뼸�η�̪��ָʾ��
C������ƽ��ȷ��ȡ�ռ���ƷW g�����ձ���������ˮ�ܽ�
D�������ʵ���Ũ��ΪM�ı�������Һװ����ϴ�õ���ʽ�ζ����У�����Һ��ʹ��ʼ����ΪV1 mL
E.����ƿ�µ�һ�Ű�ֽ���ζ�����Һǡ���ɺ�ɫ��Ϊ��ɫʱ�����¶���ΪV2 mL
����գ�
��1����ȷ���������˳���� (����ĸ��ʾ����
��2���۲�ζ���Һ��ĸ߶�ʱӦע��
��3��E����IJ�������ƿ�µ�һ�Ű�ֽ�������� ��
��4��ijѧ��ʵ��ʱ����ƿ���ռ���Ʒ��Һϴ�ӣ�ʹ�ⶨ��Ũ��_________���ƫ�ߡ���ƫ�͡�����Ӱ�족����ԭ����
��5�����ռ���Ʒ���ȵļ���ʽΪ_________________________��
��1��ȷ�����ᱻ��ȫ�к� (2��B (3���û��β������������
(4��56.85 kJ��mol-1 , H2SO4��aq����2NaOH��aq����Na2SO4��aq��+2H2O ��H=-113.7kJ��mol-1
(5������ Ũ��������ˮ�ų�����
�𰸣���1��CABDE
(2���ζ���Ҫֱ����װҺ�����1��2 min����ܹ۲�Һ��߶ȣ�����ʱ������Һ��İ�Һ�漰�̶�����ͬһˮƽ���ϣ�����Ӧȷ��0.01 mL
(3��ʹ�ζ��յ���ɫ�仯�����ԣ����ڷֱ� (4��ƫ��
���Һ��Ʒմ����ƿ�ڱ�ʹ�ռ�����ʵ������ӣ����ñ�������ƫ���ռ�Ũ��ƫ��
(5��0.8��V2��V1��M/W��100%
����


 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����֪�ⶨ�к��ȵ�ʵ�鲽�����£�����ȡ50mL 0.25mol/L���ᵹ��С�ձ��У������¶� ����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ� �۽�NaOH��Һ����С�ձ��У���Ͼ��Ⱥ�������Һ�¶ȡ���ش�
��1��NaOH��Һ�Թ�����ԭ�� ��
��2������NaOH��Һ����ȷ������ (����ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������ ��
��4������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c=4.18J��(g����)-1�������ʵ����������к���Ϊ�������� ���� д���÷�Ӧ���Ȼ�ѧ����ʽ_________
��5��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų������� ���С�ڡ��������ڡ����ڡ���57.3 kJ��ԭ�������������� ����
��ij�ռ���Ʒ�����������������õ����ʣ�Ϊ�˲ⶨ�䴿�ȣ��������²�����
A����250 mL������ƿ�ж������250 mL�ռ���Һ
B���ü�ʽ�ζ�����ȡ25.00 mL�ռ���Һ����ƿ�У������뼸�η�̪��ָʾ��
C������ƽ��ȷ��ȡ�ռ���ƷW g�����ձ���������ˮ�ܽ�
D�������ʵ���Ũ��ΪM�ı�������Һװ����ϴ�õ���ʽ�ζ����У�����Һ��ʹ��ʼ����ΪV1 mL
E.����ƿ�µ�һ�Ű�ֽ���ζ�����Һǡ���ɺ�ɫ��Ϊ��ɫʱ�����¶���ΪV2 mL
����գ�
��1����ȷ���������˳���� (����ĸ��ʾ����
��2���۲�ζ���Һ��ĸ߶�ʱӦע��
��3��E����IJ�������ƿ�µ�һ�Ű�ֽ�������� ��
��4��ijѧ��ʵ��ʱ����ƿ���ռ���Ʒ��Һϴ�ӣ�ʹ�ⶨ��Ũ��_________���ƫ�ߡ���ƫ�͡�����Ӱ�족����ԭ����
��5�����ռ���Ʒ���ȵļ���ʽΪ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ĵ��ɶ������ѧУ����10���¿���ѧ�� ���ͣ�ʵ����
����֪�ⶨ�к��ȵ�ʵ�鲽�����£�����ȡ50mL 0.25 mol/L���ᵹ��С�ձ��У������¶� ����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ� �۽�NaOH��Һ����С�ձ��У���Ͼ��Ⱥ�������Һ�¶ȡ���ش�
��1��NaOH��Һ�Թ�����ԭ�� ��
��2������NaOH��Һ����ȷ������ (����ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������ ��
��4������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c=4.18 J��(g����)-1�������ʵ����������к���Ϊ�������� ���� д���÷�Ӧ���Ȼ�ѧ����ʽ_________
��5��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų������� ���С�ڡ��������ڡ����ڡ���57.3 kJ��ԭ�������������� ����
��ij�ռ���Ʒ�����������������õ����ʣ�Ϊ�˲ⶨ�䴿�ȣ��������²�����
A����250 mL������ƿ�ж������250 mL�ռ���Һ
B���ü�ʽ�ζ�����ȡ25.00 mL�ռ���Һ����ƿ�У������뼸�η�̪��ָʾ��
C������ƽ��ȷ��ȡ�ռ���ƷW g�����ձ���������ˮ�ܽ�
D�������ʵ���Ũ��ΪM�ı�������Һװ����ϴ�õ���ʽ�ζ����У�����Һ��ʹ��ʼ����ΪV1 mL
E.����ƿ�µ�һ�Ű�ֽ���ζ�����Һǡ���ɺ�ɫ��Ϊ��ɫʱ�����¶���ΪV2 mL
����գ�
��1����ȷ���������˳���� (����ĸ��ʾ����
��2���۲�ζ���Һ��ĸ߶�ʱӦע��
��3��E����IJ�������ƿ�µ�һ�Ű�ֽ�������� ��
��4��ijѧ��ʵ��ʱ����ƿ���ռ���Ʒ��Һϴ�ӣ�ʹ�ⶨ��Ũ��_________���ƫ�ߡ���ƫ�͡�����Ӱ�족����ԭ����
��5�����ռ���Ʒ���ȵļ���ʽΪ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
����֪�ⶨ�к��ȵ�ʵ�鲽�����£�����ȡ50mL 0.25 mol/L���ᵹ��С�ձ��У������¶� ����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ� �۽�NaOH��Һ����С�ձ��У���Ͼ��Ⱥ�������Һ�¶ȡ���ش�
��1��NaOH��Һ�Թ�����ԭ�� ��
��2������NaOH��Һ����ȷ������ (����ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������ ��
��4������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c=4.18 J��(g����)-1�������ʵ����������к���Ϊ�������� ���� д���÷�Ӧ���Ȼ�ѧ����ʽ_________
��5��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų������� ���С�ڡ��������ڡ����ڡ���57.3 kJ��ԭ�������������� ����
��ij�ռ���Ʒ�����������������õ����ʣ�Ϊ�˲ⶨ�䴿�ȣ��������²�����
A����250 mL������ƿ�ж������250 mL�ռ���Һ
B���ü�ʽ�ζ�����ȡ25.00 mL�ռ���Һ����ƿ�У������뼸�η�̪��ָʾ��
C������ƽ��ȷ��ȡ�ռ���ƷW g�����ձ���������ˮ�ܽ�
D�������ʵ���Ũ��ΪM�ı�������Һװ����ϴ�õ���ʽ�ζ����У�����Һ��ʹ��ʼ����ΪV1 mL
E.����ƿ�µ�һ�Ű�ֽ���ζ�����Һǡ���ɺ�ɫ��Ϊ��ɫʱ�����¶���ΪV2 mL
����գ�
��1����ȷ���������˳���� (����ĸ��ʾ����
��2���۲�ζ���Һ��ĸ߶�ʱӦע��
��3��E����IJ�������ƿ�µ�һ�Ű�ֽ�������� ��
��4��ijѧ��ʵ��ʱ����ƿ���ռ���Ʒ��Һϴ�ӣ�ʹ�ⶨ��Ũ��_________���ƫ�ߡ���ƫ�͡�����Ӱ�족����ԭ����
��5�����ռ���Ʒ���ȵļ���ʽΪ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ĵ�ʡ�ɶ������ѧУ2010-2011ѧ�����10�µڶ����¿� ���ͣ������
����֪�ⶨ�к��ȵ�ʵ�鲽�����£�����ȡ50mL 0.25 mol/L���ᵹ��С�ձ��У������¶� ����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ� �۽�NaOH��Һ����С�ձ��У���Ͼ��Ⱥ�������Һ�¶ȡ���ش�
��1��NaOH��Һ�Թ�����ԭ�� ��
��2������NaOH��Һ����ȷ������ (����ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��3��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������ ��
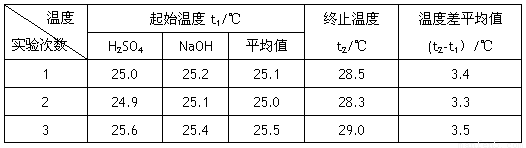
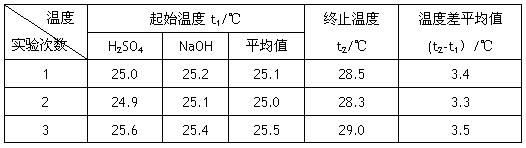
��4������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c=4.18 J��(g����)-1�������ʵ����������к���Ϊ�������� ���� д���÷�Ӧ���Ȼ�ѧ����ʽ_________
|
�¶� ʵ����� |
��ʼ�¶�t1/�� |
��ֹ�¶� t2/�� |
�¶Ȳ�ƽ��ֵ (t2-t1��/�� |
||
|
H2SO4 |
NaOH |
ƽ��ֵ |
|||
|
1 |
25.0 |
25.2 |
25.1 |
28.5 |
3.4 |
|
2 |
24.9 |
25.1 |
25.0 |
28.3 |
3.3 |
|
3 |
25.6 |
25.4 |
25.5 |
29.0 |
3.5 |
��5��������0.5 mol H2SO4��Ũ�����뺬1 mol NaOH����Һ��ϣ��ų������� ���С�ڡ��������ڡ����ڡ���57.3 kJ��ԭ������������������������������������ ����
��ij�ռ���Ʒ�����������������õ����ʣ�Ϊ�˲ⶨ�䴿�ȣ��������²�����
A����250 mL������ƿ�ж������250 mL�ռ���Һ
B���ü�ʽ�ζ�����ȡ25.00 mL�ռ���Һ����ƿ�У������뼸�η�̪��ָʾ��
C������ƽ��ȷ��ȡ�ռ���ƷW g�����ձ���������ˮ�ܽ�
D�������ʵ���Ũ��ΪM�ı�������Һװ����ϴ�õ���ʽ�ζ����У�����Һ��ʹ��ʼ����ΪV1 mL
E.����ƿ�µ�һ�Ű�ֽ���ζ�����Һǡ���ɺ�ɫ��Ϊ��ɫʱ�����¶���ΪV2 mL
����գ�
��1����ȷ���������˳���� (����ĸ��ʾ����
��2���۲�ζ���Һ��ĸ߶�ʱӦע��
��3��E����IJ�������ƿ�µ�һ�Ű�ֽ�������� ��
��4��ijѧ��ʵ��ʱ����ƿ���ռ���Ʒ��Һϴ�ӣ�ʹ�ⶨ��Ũ��_________���ƫ�ߡ���ƫ�͡�����Ӱ�족����ԭ����
��5�����ռ���Ʒ���ȵļ���ʽΪ_________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com