
| A�� | �����Һ����ˮ�������c��H+��=1��10-5mol/L | |
| B�� | c��A-��+c��HA��=2c ��Na+��=0.4 mol/L | |
| C�� | HA��Һ��$\frac{c��{A}^{-}��}{c��HA��•c��O{H}^{-}��}$�����������Һ��$\frac{c��{A}^{-}��}{c��HA��•c��O{H}^{-}��}$��� | |
| D�� | c��A-��-c��HA��=2 c ��H+��-c ��OH-�� |
���� ��0.4mol/L HA��Һ��0.2mol/LNaOH��Һ�������ϣ���Һ������Ϊ������NaA��HA����û����Һ��pH=5��HA�������NaAˮ�⣬
A�����������ԣ�����ˮ�ĵ��룻
B����Ϻ������Ϊԭ����2������������غ������
C.$\frac{c��{A}^{-}��}{c��HA��•c��O{H}^{-}��}$=$\frac{c��{A}^{-}��}{c��HA��•\frac{Kw}{c��{H}^{+}��}}$=$\frac{Ka}{Kw}$��
D���������غ��֪��c��A-��+c��HA��=2c ��Na+�����ɵ���غ��֪��c��A-��+c��OH-��=c ��H+��+c ��Na+�����Դ������
��� �⣺A�����������ԣ�����ˮ�ĵ��룬������Һ����ˮ�������c��H+��=$\frac{1{0}^{-14}}{1{0}^{-5}}$=1��10-9mol/L����A����
B��������������غ��֪c��A-��+c��HA��=2c ��Na+��=$\frac{0.4mol/L��V}{2V}$=0.2 mol/L����B����
C.$\frac{c��{A}^{-}��}{c��HA��•c��O{H}^{-}��}$=$\frac{c��{A}^{-}��}{c��HA��•\frac{Kw}{c��{H}^{+}��}}$=$\frac{Ka}{Kw}$��Ka��Kw�����¶��йأ���HA��Һ��$\frac{c��{A}^{-}��}{c��HA��•c��O{H}^{-}��}$�����������Һ��$\frac{c��{A}^{-}��}{c��HA��•c��O{H}^{-}��}$��ȣ���C��ȷ��
D���������غ��֪��c��A-��+c��HA��=2c ��Na+�����ɵ���غ��֪��c��A-��+c��OH-��=c ��H+��+c ��Na+������2c��OH-��=2 c ��H+��+c��HA������D����
��ѡC��
���� ���⿼������ϵĶ����жϣ�Ϊ��Ƶ���㣬������Һ�е����ʡ���ɼ������غ�Ϊ�����ؼ������ط�����Ӧ�������Ŀ��飬ע��ѡ��CΪ�����ѵ㣬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
 �����������ؾ��壨K3[Fe��C2O4��3]•xH2O����һ�ֹ������ϣ�110��ʱʧȥȫ���ᾧˮ��ijʵ��С��Ϊ�ⶨ�þ��������ĺ�������������ʵ�飬���������գ�
�����������ؾ��壨K3[Fe��C2O4��3]•xH2O����һ�ֹ������ϣ�110��ʱʧȥȫ���ᾧˮ��ijʵ��С��Ϊ�ⶨ�þ��������ĺ�������������ʵ�飬���������գ�| �ζ����� | �ζ���ʼ������mL�� | �ζ��յ������mL�� |
| ��һ�� | 1.08 | ����ͼ |
| �ڶ��� | 2.02 | 24.52 |
| ������ | 1.00 | 20.98 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCO3-+H2O?H3O++CO32- | B�� | HS-+H2O?H2S+OH- | ||
| C�� | Fe3++3H2O?Fe��OH��3��+3H+ | D�� | Br-+H2O?HBr+OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���� | �۵�/�� | �е�/�� |
| N2O5 | 41 | 32 �������� |
| N2O4 | -11 | 24 |
 ����Է�������137�����������£���������ƿ�з��������30mL N2O5��CH2Cl2��Һ��N2O5��Ũ��Ϊlmol•L-1����30��ʱ���μ�15mL�ױ�����ַ�Ӧ�ö������ױ� 1.73g���ش��������⣺
����Է�������137�����������£���������ƿ�з��������30mL N2O5��CH2Cl2��Һ��N2O5��Ũ��Ϊlmol•L-1����30��ʱ���μ�15mL�ױ�����ַ�Ӧ�ö������ױ� 1.73g���ش��������⣺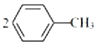 +N2O5$��_{��}^{����}$
+N2O5$��_{��}^{����}$ +H2O��
+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��| ���� | ���볣����Ka�� |
| HCN | Ka=5��10-10 |
| H2CO3 | Ka1=4.5��10-7 Ka2=4.7��10-11 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����ͼ��ʾװ�ü�����������ʱ�����ܴﵽĿ���ǣ�������
����ͼ��ʾװ�ü�����������ʱ�����ܴﵽĿ���ǣ�������| ѡ�� | ���ɵ����� | �Լ�X | �Լ�Y |
| A | ��ʯ��ˮ��Ӧ��ȡ����Ȳ | CuSO4��Һ | ��ˮ |
| B | ľ̿��ŨH2SO4���ȵõ��Ķ�����̼ | ����NaHCO3��Һ | ����ʯ��ˮ |
| C | CH3CH2Br��NaOH����Һ���ȵ� ������ϩ | ˮ | KMnO4 ������Һ |
| D | C2H5OH��ŨH2SO4������170����ȡ����ϩ | NaOH��Һ | ��ˮ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 Ũ�Ⱦ�Ϊ0.1mol•L-1�������ΪV0��HX��HY��Һ���ֱ��ˮϡ�������V��pH��lg$\frac{v}{{v}_{0}}$�ı仯��ϵ��ͼ��ʾ������������ȷ���ǣ�������
Ũ�Ⱦ�Ϊ0.1mol•L-1�������ΪV0��HX��HY��Һ���ֱ��ˮϡ�������V��pH��lg$\frac{v}{{v}_{0}}$�ı仯��ϵ��ͼ��ʾ������������ȷ���ǣ�������| A�� | �����£���ˮ�������c��H+��•c��OH -����a��b | |
| B�� | HX��HY�������ᣬ��HX�����Ա�HY���� | |
| C�� | ��ͬ�¶��£����볣��K�� HX����a��b | |
| D�� | lg$\frac{v}{{v}_{0}}$=3����ͬʱ��������Һ��������HX��HY��H2O�Ļӷ�������$\frac{c��{X}^{-}��}{c��{Y}^{-}��}$��С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ����Ļ�ѧʽ | CH3COOH | HCN | H2S |
| ���볣�� | 1.8��10-5 | 4.9��10-10 | K1=9.1��10-8 K2=1.1��10-12 |
| A�� | �����ʵ���Ũ�ȵĸ���Һ pH ��ϵΪ��pH��Na2S����pH��NaCN����pH��NaHS����pH��CH3COONa�� | |
| B�� | �� 0.1mol/L �� NaOH ��Һ�ζ� pH ֵ��ȵ�CH3COOH�� HCN ��Һ��CH3COOH���ĵ�NaOH��Һ��� ���� | |
| C�� | NaHS ��Na2S �Ļ����Һ�У�һ������c��Na+��+c��H+��=c��OH-��+c��HS-��+2c��S2-��+c��H2S�� | |
| D�� | ijŨ�ȵ�NaCN��Һ�� pH=d����������ˮ�������c��OH-��=10-4mol/L |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com