
|
��֪��Ӧ��H2(g)��
��Ӧ2NH3(g)�� | |
| [����] | |
A�� |
2��H1��2��H2��2��H3 |
B�� |
��H1����H2����H3 |
C�� |
3��H1��2��H2��2��H3 |
D�� |
3��H1��2��H2��2��H3 |
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.H2��ȼ����Ϊ184.6kJ��mol
B.1mol H��H��lmolCl��Cl�ļ���֮��С��2mol H��Cl���ļ���֮��
C.ϡH2SO4��ϡNaOH��Һ��Ӧ���к���Ϊ-57.3kJ��mol
D.ϡ������ϡNaOH��Һ��Ӧ����1molH2O���ų�57.6kJ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ������һ�и߶���һ���¿���ѧ�Ծ����������� ���ͣ�������
(6��)���ø�˹���ɽ�����и�С��
(1)��֪��Ӧ��H2(g)+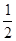 O2(g)==H2O(g) ��H1 N2(g)+2O2==2NO2(g) ��H2
O2(g)==H2O(g) ��H1 N2(g)+2O2==2NO2(g) ��H2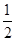 N2(g)+
N2(g)+ H2(g)==NH3(g) ��H3
H2(g)==NH3(g) ��H3
��������������Ӧ������4NH3(g)+7O2(g)==4NO2(g)+6H2O(g)�ķ�Ӧ�ʱ�Ϊ (�ú���H1����H2����H3��ʽ�ӱ�ʾ)��
(2) ��֪��CH4(g)��H2O(g)===CO(g)��3H2(g) ��H��+206.2 kJ��mol��1
CH4(g)��CO2(g)===2CO(g)��2H2(g) ��H��+247.4 kJ��mol��1
���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�������ʡ������ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪��Ӧ��H2(g) ��
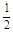 O2(g) �� H2O(g)
��H1
O2(g) �� H2O(g)
��H1
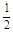 N2(g) �� O2(g) �� NO2
(g) ��H2
N2(g) �� O2(g) �� NO2
(g) ��H2
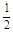 N2(g) ��
N2(g) ��
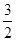 H2(g) �� NH3 (g) ��H3
H2(g) �� NH3 (g) ��H3
��Ӧ2NH3
(g) ��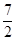 O2(g) ��2NO2 (g) ��3H2O(g)
�ġ�H��
O2(g) ��2NO2 (g) ��3H2O(g)
�ġ�H��
A��2��H1��2��H2��2��H3 B����H1����H2����H3
C��3��H1��2��H2��2��H3 D��3��H1��2��H2��2��H3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�����и߶���һ���¿���ѧ�Ծ��������棩 ���ͣ�������
(6��)���ø�˹���ɽ�����и�С��
(1)��֪��Ӧ��H2(g)+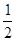 O2(g)==H2O(g)
��H1
N2(g)+2O2==2NO2(g) ��H2
O2(g)==H2O(g)
��H1
N2(g)+2O2==2NO2(g) ��H2
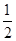 N2(g)+
N2(g)+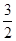 H2(g)==NH3(g)
��H3
H2(g)==NH3(g)
��H3
��������������Ӧ������4NH3(g)+7O2(g)==4NO2(g)+6H2O(g)�ķ�Ӧ�ʱ�Ϊ (�ú���H1����H2����H3��ʽ�ӱ�ʾ)��
(2) ��֪��CH4(g)��H2O(g)===CO(g)��3H2(g) ��H��+206.2 kJ��mol��1
CH4(g)��CO2(g)===2CO(g)��2H2(g) ��H��+247.4 kJ��mol��1
���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com