
����Ŀ�������������ɲ⣬��������仯�����ʵ������ṹ�����˱仯�Ľ�����ش�����������
��1�������ӻ���������ж������з��ӵĿռ乹����V�ε���_____(����ĸ)��
A.BeCl2 B.H2O C.HCHO D.CS2
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��м�λ��ͬһ������λ��ͬһ������T��ԭ��������Q��2��T�Ļ�̬ԭ�ӵ���Χ����(�۵���)�Ų�ʽΪ____��Q2+��δ�ɶԵ�������_____��
��3��ͭ����Ͻ�����������ʹ�õĽ���������Cu2+����NH3�γ���λ��Ϊ4�������[Cu(NH3)4]SO4��
����Ԫ�������ڱ��е�λ����______��[Cu(NH3)4]SO4����N��O��S����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ______��
��[Cu(NH3)4]SO4�У����ڵĻ�ѧ����������______(����ĸ)��
A���Ӽ� B������ C.��λ�� D.�Ǽ��Լ� E���Լ�
��NH3��Nԭ�ӵ��ӻ����������______��д��һ����SO42-��Ϊ�ȵ�����ķ��ӵĻ�ѧʽ:_ ______��
��[Cu(NH3)4]2+���жԳƵĿռ乹����[Cu(NH3)4]2+�е�����NH3������Cl-ȡ�����ܵõ����ֲ�ͬ�ṹ�IJ���.��[Cu(NH3)4]2+�Ŀռ乹��Ϊ_________��
��4������ͭ�γɵ�ij�����Ӿ���ľ�����ͼ��ʾ����û�����Ļ�ѧʽΪ_____������þ�����ܶ�Ϊ��g/cm3����þ�����ͭ�����������Ӽ���������Ϊ_____(�ú����Ĵ���ʽ��ʾ�����а����ӵ�������NA��ʾ)cm��

���𰸡� B 3d84s2 4 ��������IB�� N>O>S ACE sp3 CCl4(������������) ƽ�������� CuO 
����������1��BeCl2����������ԭ�Ӻ����������۵����������µ��Ӷ������Լ۲���Ӷ�����2������ԭ����sp�ӻ�����ɼ������ӵ����幹��Ϊֱ������A������ˮ�����йµ��Ӷ���![]() ��ˮ������ԭ�Ӻ���2�����۵��������Լ۲���Ӷ�����4������ԭ����
��ˮ������ԭ�Ӻ���2�����۵��������Լ۲���Ӷ�����4������ԭ����![]() �ӻ�����ɼ����۲���ӶԻ���ģ��Ϊ��������������2�Թ¶Ե��������ӵ����幹��ΪV����B��ȷ�� HCHO������
�ӻ�����ɼ����۲���ӶԻ���ģ��Ϊ��������������2�Թ¶Ե��������ӵ����幹��ΪV����B��ȷ�� HCHO������![]() ̼ԭ���γ�3��
̼ԭ���γ�3��![]() �����¶Ե����������м۲���ӶԸ���
�����¶Ե����������м۲���ӶԸ���![]() ���ӻ���ʽΪ
���ӻ���ʽΪ![]() �ӻ����۲���ӶԻ���ģ��Ϊƽ����������û�йµ��Ӷ������ӵ����幹��Ϊƽ����������C����������̼������̼ԭ�Ӻ���2��
�ӻ����۲���ӶԻ���ģ��Ϊƽ����������û�йµ��Ӷ������ӵ����幹��Ϊƽ����������C����������̼������̼ԭ�Ӻ���2��![]() ���Ҳ����µ��Ӷ�������sp�ӻ�����ռ乹����ֱ������D��������ȷѡ��B��
���Ҳ����µ��Ӷ�������sp�ӻ�����ռ乹����ֱ������D��������ȷѡ��B��
(2) ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��Q��T���ڵ����壬��ԭ������T��Q��2����QΪFeԪ�أ�TΪNiԪ�أ�NiԪ����28��Ԫ�أ�Niԭ�Ӽ۵����Ų�ʽΪ3d84s2��Fe2+�ĺ�������Ų�ʽΪ1s24s22p63s23d6��3d�ܼ���4��δ�ɶԵ��ӣ���ȷ����3d84s2 ��4 ��
(3) ��ͭԪ�غ˵����Ϊ29�������ڱ��е�λ���ǵ�������IB�壻N��O����ͬһ���ڣ�����Nԭ�ӵ�2p������ڰ����״̬���ʵ�һ������N>O����O��S��ͬһ���壬ͬ����Ԫ�صĵ�һ�����ܴ��ϵ������μ�С���ʵ�һ������O>S����N>O>S����ȷ����N>O>S��
��[Cu(NH3)4]SO4�У�SO2-4��[Cu(NH3)4]2+��������Ӽ���Nԭ�Ӻ�Cuԭ�Ӽ������λ����N��H����S��O��Ϊ���Լ���ѡA��C��E����ȷ����A��C��E��
��NH3��Nԭ�ӵļ۵��Ӷ�����![]() (5��1��3)��3��4���ʲ�ȡsp3�ӻ���ʽ����SO2-4��Ϊ�ȵ�����ķ��ӵĻ�ѧʽΪCCl4��SiCl4������ȷ����sp3 �� CCl4��
(5��1��3)��3��4���ʲ�ȡsp3�ӻ���ʽ����SO2-4��Ϊ�ȵ�����ķ��ӵĻ�ѧʽΪCCl4��SiCl4������ȷ����sp3 �� CCl4��
��[Cu(NH3)4]2+���жԳƵĿռ乹�ͣ�[Cu(NH3)4]2+�е�����NH3������Cl��ȡ�����ܵõ����ֲ�ͬ�ṹ�IJ����[Cu(NH3)4]2+�Ŀռ乹��Ϊƽ������������ȷ����ƽ�������Ρ�
(4) �ɾ�̯��֪��1�������к�4��ͭ���ӣ�������Ϊ8��![]() +1+2��
+1+2��![]() +4��
+4��![]() =4������û�����Ļ�ѧʽΪCuO��������ͭ�����������Ӽ���������Ϊ������Խ��߳���
=4������û�����Ļ�ѧʽΪCuO��������ͭ�����������Ӽ���������Ϊ������Խ��߳���![]() ���辧���ı߳�Ϊacm��ƽ��1����������m=
���辧���ı߳�Ϊacm��ƽ��1����������m=![]() ��80 g�����V=a3cm3��������=
��80 g�����V=a3cm3��������=![]() ���ɽ��a=
���ɽ��a=![]() ��������ͭ�����������Ӽ������������
��������ͭ�����������Ӽ������������![]() a=
a=![]() (cm)����ȷ����
(cm)����ȷ����![]() ��
��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��
2H2(g����O2(g��===2H2O(l�� ��H����571.6 kJ��mol��1
2CH3OH(l����3O2(g��===2CO2(g����4H2O(l����H����1452 kJ��mol��1
H��(aq����OH��(aq��===H2O(l�� ��H����57.3 kJ��mol��1
����˵����ȷ���� �� ��
A. H2(g����ȼ����Ϊ571.6 kJ��mol��1
B. ͬ������H2(g����CH3OH(l����ȫȼ�գ�H2(g���ų���������
C. ![]() H2SO4(aq����
H2SO4(aq����![]() Ba(OH��2(aq��===
Ba(OH��2(aq��===![]() BaSO4(s����H2O(l�� ��H����57.3 kJ��mol��1
BaSO4(s����H2O(l�� ��H����57.3 kJ��mol��1
D. 3H2(g����CO2(g��=CH3OH(l����H2O(l�� ��H����135.9 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25 ������c(CH3COOH)��c(CH3COO��)��0.1 mol��L��1��һ�����ʹ����ƻ����Һ����Һ��c(CH3COOH)��c(CH3COO��)��pH�Ĺ�ϵ��ͼ��ʾ�������й���������ȷ����(����)

A. pH��5.5��Һ�У�c(CH3COOH)>c(CH3COO��)��c(H��)��c(OH��)
B. ��ͼ��֪���¶��´���ĵ��볣��Ϊ1��10��4.75
C. pH��3.5��Һ�У�c(Na��)��c(H��)��c(OH��)��c(CH3COOH)��0.1 mol��L��1
D. ��1 LW������ʾ��Һ��ͨ��0.05 mol HCl����(��Һ����仯�ɺ���)��2c(H��)��c(CH3COOH)��c(CH3COO��)��2c(OH��)��2c(Cl��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO��CO2�ǻ�ʯȼ��ȼ�յ���Ҫ���
(1)����002molCO2��001molCO�Ļ������ͨ��������Na2O2������ܱ������У�ͬʱ���ϵ��õ��ȼ����ַ�Ӧ������������______g��
(2)��֪:2CO(g)+O2(g)=2CO2(g) ��H=-566.0kJ/mol������Eo-o=499.0kJ/mol��
�ٷ�Ӧ:CO(g)+O2(g)![]() CO2(g)+O(g)�ġ�H=______kJ/mol��
CO2(g)+O(g)�ġ�H=______kJ/mol��
����֪2500Kʱ�����з�Ӧ��ƽ�ⳣ��Ϊ0.40��ijʱ�̸÷�Ӧ��ϵ�и�����Ũ������:c(CO)��c(O2)=c(CO2) ��c(O)�����ʱv(��)_____(�>""<"��"=��)v(��)��
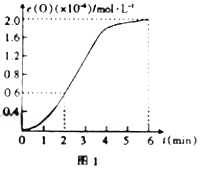
����֪1500��ʱ�����ܱ������з�����Ӧ:CO2(g)![]() CO(g)+O(g)����Ӧ������O(g)�����ʵ���Ũ����ʱ��ı仯��ͼ1��ʾ����0~2min�ڣ�CO2��ƽ����Ӧ����v(CO2)=______��
CO(g)+O(g)����Ӧ������O(g)�����ʵ���Ũ����ʱ��ı仯��ͼ1��ʾ����0~2min�ڣ�CO2��ƽ����Ӧ����v(CO2)=______��
(3)��ij�ܱ������з�����Ӧ��2CO2(g)![]() 2CO(g)+O2(g),1molCO2�ڲ�ͬ�¶��µ�ƽ��ֽ�����ͼ2��ʾ��
2CO(g)+O2(g),1molCO2�ڲ�ͬ�¶��µ�ƽ��ֽ�����ͼ2��ʾ��
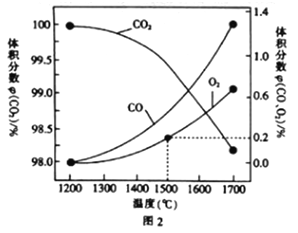
�ٺ��º��������£��ܱ�ʾ�ÿ��淴Ӧ�ﵽƽ��״̬����___(����ĸ).
A.CO������������ֲ���
B.�����ڻ��������ܶȱ��ֲ���
C.�����ڻ�������ƽ��Ħ���������ֲ���
D.��λʱ���ڣ�����CO��Ũ�ȵ�������CO2��Ũ��
�ڷ���ͼ2����1500��ʱ��Ӧ�ﵽƽ��״̬�����������Ϊ1L�����ʱ��Ӧ��ƽ�ⳣ��K=____(����������1λС��)��
����2L�ĺ����ܱ������г���2molCO2(g)��������Ӧ��2CO2(g)![]() 2CO(g)+O2(g)������¶�ΪT��ʱ��������O2�����ʵ���Ũ����ʱ��ı仯������II��ʾ��ͼ������I��ʾ���������II���ı�һ�ַ�Ӧ������c(O2)��ʱ��ı仯����ı��������______��a��b������COŨ�ȱ仯��ʾ�ľ���Ӧ���ʹ�ϵΪva(CO)_____(�>����<����=��)vb(CO)��
2CO(g)+O2(g)������¶�ΪT��ʱ��������O2�����ʵ���Ũ����ʱ��ı仯������II��ʾ��ͼ������I��ʾ���������II���ı�һ�ַ�Ӧ������c(O2)��ʱ��ı仯����ı��������______��a��b������COŨ�ȱ仯��ʾ�ľ���Ӧ���ʹ�ϵΪva(CO)_____(�>����<����=��)vb(CO)��
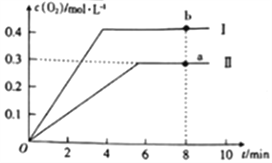
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����״���£�22.4LH2������Ϊ____________g��
��2��9.8g H2SO4�����ʵ���Ϊ__________mol��Լ����___________����ԭ�ӡ�
��3��VL Fe2(SO4)3��Һ�к���ag SO42-,ȡ����Һ0.5L����ˮϡ����2VL,��ϡ�ͺ����Һ��Fe3+�����ʵ���Ũ��Ϊ_________mol/L��
��4����״���£�16 gij��̬������RO2�����Ϊ5.6 L���������Ħ��������________��
��5��b%��������Һ��4b%��������Һ�������Ϻ�������Һ����������______���� �������� = ��ͬ��2.5b%��
��6����ij�¶�ʱ��һ������Ԫ��A����̬�⻯��AH3����һ��������ܱ���������ȫ�ֽ���������嵥�ʣ���ʱѹǿ������75%������A�Ļ�ѧʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ŷ����λ��ѧ���������ӻ����������ϳ����о����ٻ�2016��ŵ������ѧ���������ӻ����о������г������������������������ͼ��ʾ������˵����ȷ����

A. �٢ۻ�Ϊͬϵ�� B. �٢ڢܾۢ�������
C. �٢ܵ�һ�ȴ����Ϊ���� D. �ڢܻ�Ϊͬ���칹��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ��װ��(I)��һ�ֿɳ���أ�װ��(��)Ϊ���Ե缫�ĵ��ء�����˵����ȷ����

A. �պϿ���Kʱ���缫BΪ�������ҵ缫��ӦʽΪ��2Br����2e��=Br2
B. װ��(I)�ŵ�ʱ���ܷ�ӦΪ��2Na2S2+Br2=Na2S4+2NaBr
C. װ��(I)���ʱ��Na+������ͨ�������ӽ���Ĥ
D. ��װ�õ�·����0.1mole��ͨ��ʱ���缫��������3.2gCu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����㲢��գ����ڱ�״����15gCO��CO2�Ļ�����壬���Ϊ11.2L����
��1����������ƽ��Ħ�������� ___________________g/mol
��2��CO�����������___________________
��3��CO2��CO���������� ____________________________
��4���������������̼ԭ�ӵ������� ___________________ g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У���ȷ���ǣ� ��
A. Ԫ�����ڱ�ÿһ����Ԫ��ԭ�ӵ����������Ų����Ǵ�ns1 ���ɵ�ns2np6
B. ��ij��̬ԭ�ӵ���Χ�����Ų�Ϊ4d25s2�����ǵ�������IVB��Ԫ��
C. M��ȫ������N��Ϊ4s1��ԭ�Ӻ�λ�ڵ������ڵ���A���ԭ����ͬһ��Ԫ��
D. ��ԭ����1s22s22p63s1��1s22s22p63p1ʱ��ԭ���ͷ��������ɻ�̬ת���ɼ���̬
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com