
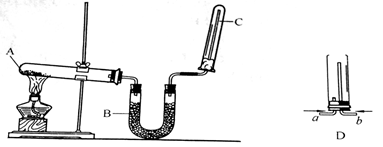
 2N2+6H2O
2N2+6H2O 2N2+6H2O��
2N2+6H2O��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������Ӧʱ����Ҫ�ǣ�5�۵ĵ��õ��� |
| B��ŨHNO3��ŨHCl��3��1����������õĻ�������ˮ |
| C������������H�����ӣ��ܱ�Zn��Fe�Ƚ�����ԭ��H2 |
| D�������£���ŨHNO3��Ͷ��FeƬ������������ĺ���ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH3��NO | B��NO��NO2 | C��NH3��NH4NO3 | D��N2��NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Ӧ��ÿ����lmolN2O������67.2LCO |
| B�������ʵ�����N2O��CO2������ȵĵ����� |
| C��N2Oֻ�������ԣ���ԭ�� |
| D��N2O��Ѹ��������Ѫ�쵰��ϣ�ʹ���ж� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO������ijЩ���ͼ�N���������IJ�� |
| B��NO����һ�ִ�����Ⱦ����Ѫ�쵰��ʹ���ж��� |
| C��NO������β�������ŷŲ�������Ⱦ֮һ�� |
| D��NO�Ǻ���ɫ���壻 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| B����NaOH��Һ�����ȣ�������ɫʯ���Լ� |
| C����NaOH��Һ�����ȣ������̪�Լ� |
| D����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
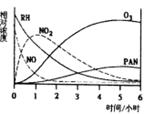
| A��NO����ʧ�����ʱ�RH�� | B��NOת��ΪNO2 |
| C��RH��NO2ת��ΪPAN��O3 | D��O3ת��ΪPAN |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com