
| A�� | ����Һ��һ������SO42-��NH4+��CO32-��Cl-������ | |
| B�� | ����Һ�����ٺ���0.23g Na+ | |
| C�� | ����Һ�п��ܺ���Mg2+��Cl- | |
| D�� | ����Һ�к���0.355g Cl- |
���� ��ɫ��Һһ������Fe2+��
��ȡ������Һ���������Ba��OH��2��Һ�����ȣ�����4.30g��ɫ������0.51g�̼�����ζ����ų���������Ϊ��������NH4+Ϊ$\frac{0.51g}{17g/mol}$=0.03mol��
��ȡ�����ϲ���Һ���ȼ����ᣬ�ټ���һ��������������Һ������1.435g����������ΪAgCl����һ��Cl-��Ϊ$\frac{1.435g}{143.5g/mol}$=0.01mol��
��ȡ���й��������������ᣬ����ʣ��2.33g����һ����SO42-Ϊ$\frac{2.33g}{233g/mol}$=0.01mol����̼�ᱵΪ4.30g-2.33g=1.97g����һ����CO32-Ϊ$\frac{1.97g}{197g/mol}$=0.01mol����ԭ��Һһ��������Mg2+����ϵ���غ������
��� �⣺A��������������֪������Һ��һ������SO42-��NH4+��CO32-��Cl-�����ӣ���A��ȷ��
B���ɵ���غ��֪��0.01+0.01��2+0.01��2��0.03����֪���ٺ�0.02molNa+������Ϊ23g/mol��0.02mol=0.46g����B����
C������Һ�в�����Mg2+����C����
D��Cl-��Ϊ0.01mol��������Ϊ35.5g/mol��0.01mol=0.355g����D��ȷ��
��ѡAD��
���� ���⿼�����ӵļ��飬Ϊ��Ƶ���㣬�������ӵ����ʡ������ķ�Ӧ�����ʵ�������Ϊ�����Ĺؼ������ط�����Ӧ�������Ŀ��飬ע�����غ��Ӧ�ã���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{ab}{23}$mol | B�� | $\frac{a��b+1��}{23}$mol | C�� | $\frac{18ab}{23}$g | D�� | $\frac{18a��b+1��}{23}$mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Զ��ױ������Ը��������Һ������Ӧ�����������Ը��������Һ����Ӧ | |
| B�� | �ױ���Ũ�����Ũ������Һ�����ȣ�����Ũ�����Ũ������Һ������ | |
| C�� | �ұ��������ڴ��������¼��ȷ�Ӧ�����������ڴ��������¼��ȷ�Ӧ | |
| D�� | �ڶ��ױ��������ڹ����·�����Ӧ�������ڹ�����������������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
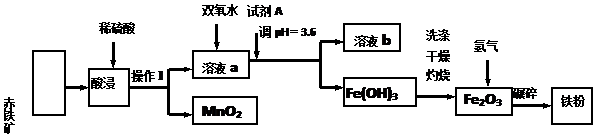
| ������ | Fe��OH��3 | Al��OH��3 | Fe ��OH��2 | Cu ��OH��2 |
| pH | 3.4 | 5.2 | 9.7 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH��ͬ�Ģ�NH4Cl��NH4Al��SO4��3��NH4HSO3��������Һ�е�c��NH4+�����٣��ڣ��� | |
| B�� | 20mL0.1mol/L��CH3COONa��Һ��10mL0.1mol/L��HCl��Һ��Ϻ�����ԣ�������Һ�У�c��CH3COO-����c��Cl-����c��CH4COOH����c��H+�� | |
| C�� | 0.1mol/L��NaHCO3��Һ��0.1mol/L��NaOH��Һ�������ϣ�������Һ�У�c��Na+����c��CO32-����c��HCO3-����c��OH-�� | |
| D�� | �����£�pH=7��NH4Cl�백ˮ�Ļ����Һ�У�c��Cl-����c��NH4+����c��H+��=c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2SO4��BaC12��K2CO3��KNO3 | B�� | HC1��NaNO3��Na2CO3����NH4��2SO4 | ||
| C�� | NaOH��KC1��K2CO3��MgCl2 | D�� | NaAlO2��NaC1��Na2CO3��AgNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
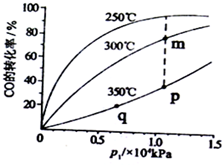 ͨ����ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ�ϣ���ҵ����CO��H2�ϳ�CH3OH����ش��������⣮
ͨ����ѧ�ӹ�ʹúת��Ϊ���塢Һ�塢����ȼ�ϣ���ҵ����CO��H2�ϳ�CH3OH����ش��������⣮| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ���� ��ʱ��/min | ||
| H2O | CO��g�� | H2 | CO��g�� | |||
| 1 | 650 | 2 | 4 | 1.6 | 2.4 | 5 |
| 2 | 900 | 1 | 2 | 0.5 | 1.5 | 2 |
| 3 | 2 | 4 | 1.0 | n | t | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com