
������֪��Ŀǰ�������ʼ��ѹ㷺����ʳƷ���ʡ���ʳҩ�ĵķ��棬��ù��������������Ӧ���������������ʣ���ȫ���հ�װ�ڵ��������Ӷ���װ�ڵ���Ʒ�������������á�����������ɺ���ɫʱ������ʧЧ�����±���һ���������������ʼ����䷽��
| ��Ҫԭ�� | ���� |
| ��̼4%�������� ��ʯ�� ʳ�� �ƾ� | 80% 20% 4g ���� |
���з�Ӧʽ��������ԭ���ص��ǣ� ��
A. Fe-2e-=Fe2+ B. C+O2=CO2
C.4Fe(OH)2+O2+2H2O=4Fe(OH)3 D. 2Fe(OH)3=Fe2O3?3H2O

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �¶ȣ��棩 | 60 | 240 | 930 | 1000 |
| ��������������g�� | 19.7 | 16.1 | 8.1 | 8.1 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
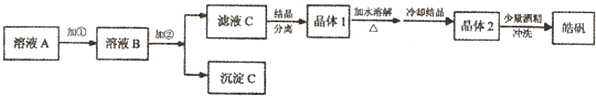
| ʵ����� | ʵ������ | ������ͣ������ӷ���ʽ��ʾ�� | |
| ̽���� | ��3�� |
��ҺpH=8 | ��4�� |
| ̽���� | ��������ˮ��pH��2���еμ�����Na2S2O3��Һ | ��ˮ��ɫ��dz | ��5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
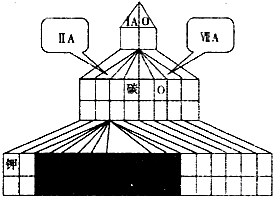
����A��B��C��D��E��F���ֶ���������Ԫ�أ���ԭ����������������֪A��Dλ��ͬһ���壬AԪ����Ԫ�����ڱ���ԭ�Ӱ뾶��С��C�Ƿǽ�������ǿ��Ԫ�أ�B��Eԭ��������������ȣ���B��Eԭ������֮����A��Dԭ������֮�͵�������F������������Ӧ��ˮ����������ǿ��
��1��������ͼ��ʽԪ�����ڱ���Ԫ�����ڱ�����һ�ֻ�������Ӧλ���б��BԪ�ص�Ԫ�ط��ţ�ͬʱ��ͼ�н�����Ԫ��Ϳ�ڡ�
��2��DԪ�صļ����ӵ����ӽṹʾ��ͼ
��3��B��E�γɵ���̬�⻯����۷е��Ǹ���____________��(�þ���Ļ�ѧʽ��ʾ)��
��4��E��C��F�����γ�һЩ��������й�����E��C���γɻ�����EC6��E����F���γɻ�����E2F2����ش��������⣺
�ټ�ͬѧͨ��������ΪEC6�����ʲ�������O2��ȼ�գ�ԭ���ǣ� ��
����ͬѧͨ��������֪������E2F2�мȴ��ڼ��Լ����ִ��ڷǼ��Լ�����д�������ʽ�� ����������ˮ���ȶ�������һ�ֻ�ɫ��������ɫ���壬���õ�һ��������Һ����д���ù��̵ķ�Ӧ����ʽ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com