
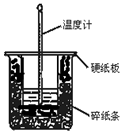
 50mL 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ��ͼʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش������⣺
50mL 0.50mol•L-1������50mL 0.55mol•L-1NaOH��Һ��ͼʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش������⣺���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ��
��4��������ʵ������ȣ�����ϡ��ǿ����ǿ�Ӧ�ⶨ�к��ȣ��Դ������
��5�������Ȼ�ѧ����ʽ����дԭ��д������ȼ��������̬ˮ���Ȼ�ѧ����ʽ������m=nM����1molˮ������Ϊ18g����������1mol��̬ˮת����Һ̬ˮ�ų������������������������Ӧ������̬ˮ�ķ�Ӧ�ȼ�������Һ̬ˮ�ķ�Ӧ�ȣ�
��� �⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������
�ʴ�Ϊ�����β����������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮��������ֽ���������Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��3�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
��4��һˮ�ϰ��ĵ������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������ƫС�����к��ȵ���ֵ��ƫС���ʴ�Ϊ��ƫС��
��5��������������Ӧ����1molˮ��������241.8kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-241.8kJ/mol��1gˮ����ת����Һ̬ˮ����2.444kJ����18gˮ����ת����Һ̬ˮ�ų�����2.444kJ��18=44kJ���ʷ�ӦH2��g��+$\frac{1}{2}$O2��g���TH2O��l���ķ�Ӧ�ȡ�H=-��241.8kJ/mol+44kJ/mol��=-285.8kJ/mol��
�ʴ�Ϊ��H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-241.8kJ/mol��-285.5��
���� ���⿼��ѧ���й��к��ȵIJⶨ���Ȼ�ѧ����ʽ����д�뷴Ӧ�ȵļ���֪ʶ�����Ը�����ѧ֪ʶ���лش��ѶȲ���


 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 $��_{��}^{Cl_{2}������}$
$��_{��}^{Cl_{2}������}$ $��_{��}^{NaOH���Ҵ�����}$A$��_{��}^{��ˮ}$B$\stackrel{��}{��}$
$��_{��}^{NaOH���Ҵ�����}$A$��_{��}^{��ˮ}$B$\stackrel{��}{��}$ $��_{��}^{Br_{2}������}$
$��_{��}^{Br_{2}������}$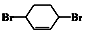 $\stackrel{��}{��}$
$\stackrel{��}{��}$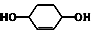
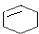 ��B�Ľṹ��ʽ��
��B�Ľṹ��ʽ��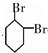 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Ca2+��Al3+��HCO3-��Cl- | B�� | K+��Ba2+��Cl-��NO3- | ||
| C�� | Na+��AlO2-��OH-��SO42- | D�� | Na+��NH4+��Cl-��K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH[H2O] | B�� | CuCl2[CuSO4] | C�� | NaCl[NaCl] | D�� | CuSO4[Cu��OH��2] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
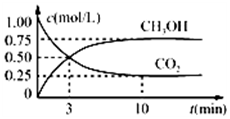 �����Ϊ1L�ĺ����ܱ������У�����1mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�������CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������
�����Ϊ1L�ĺ����ܱ������У�����1mol CO2��3mol H2��һ�������·�����Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�������CO2��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �û�ѧ��Ӧ��3 minʱ�ﵽƽ��״̬ | |
| B�� | ���������������䣬�����¶ȣ�ƽ��ʱc��CH3OH��=0.85 mol•L-1����÷�Ӧ���� | |
| C�� | ����ͬ�¶��£����������ݻ��ɱ䣬����������ѹǿ���䣬ͬ���������г���1 molCO2��3mol H2����ƽ��ʱCO2��Ũ����ͼ����ͬ | |
| D�� | 12 minʱ���������������ٳ���0.25 mol CO2��0.25 mol H2O��g������ʱ��Ӧ�����淴Ӧ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ѧ�� | N��O | Cl-Cl | Cl-N | N�TO |
| ����/kJ•mol-1 | 630 | 243 | a | 607 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��ѧ�� | N-H | N-N | N��N | O-H |
| E/��kJ•mol-1�� | 390 | 190 | 946 | 460 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com