
ˮ�ĵ���ƽ��������ͼ��ʾ��
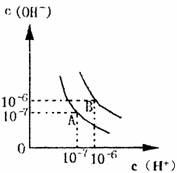
��1������A���ʾ25��ˮ�ڵ���ƽ��ʱ������Ũ�ȣ����¶�������100��ʱ��ˮ�ĵ���ƽ��״̬��B�㣬���ʱˮ�����ӻ��� ���ӵ� �����ˮ�����ӻ������ԭ���� ___________________
��
��2����֪��25��ʱ��0.1mol/L��H2R��Һ��0.7<pH<1����֪1g2=0.3������H2R��ˮ��Һ�еĵ��뷽��ʽΪ
��3��100��ʱ����pH=9��NaOH��Һ��pH=4��������Һ��ϣ���������ҺpH=7����NaOH��Һ��������Һ�������Ϊ ��
��4��100��ʱ����10�����ijǿ����Һ��1�����ijǿ����Һ��Ϻ���Һ�����ԣ�����֮ǰǿ����Һ��pH��ǿ����Һ��pH֮��Ӧ����Ĺ�ϵ�� ____________________________
_____ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ˮ�ĵ���ƽ��������ͼ��ʾ��
ˮ�ĵ���ƽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2006?�ɶ�ģ�⣩ˮ�ĵ���ƽ��������ͼ��ʾ��
��2006?�ɶ�ģ�⣩ˮ�ĵ���ƽ��������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?����һģ����1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��Cʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�100��ʱ1mol?L-1 ��NaOH��Һ�У���ˮ�������c��H+��=
��2012?����һģ����1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��Cʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�100��ʱ1mol?L-1 ��NaOH��Һ�У���ˮ�������c��H+��=| ��ѧʽ | ���볣����25�棩 |
| HCN | K=4.9��l0-10 |
| CH3COOH | K=1.8��l0-5 |
| H2CO3 | K1=4.3��l0-7��K2=5.6��l0-11 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com