
| A�� | �������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ��֧�ܿڴ� | |
| B�� | ����ƿ��©�IJ����ǣ�������ƿ��ע��������ˮ�����ϲ���ƿ����������ָ��סƿ�ף�����ʳָ��סƿ�������ã��۲��Ƿ�©ˮ | |
| C�� | �ڷ�Һ©���з�������Һ��ʱ��Ҫ�ȴ��¶˷ų��ܶȽϴ��Һ�壬�رջ�����ȡ��һֻ�ձ�������ٷų��ܶȽ�С��Һ�� | |
| D�� | ��������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ���� |
���� A������ʱ���¶ȼƲⶨ��ֵ��¶ȣ�
B����©ʱ��װˮ�����ã���������ת180�㣬�ظ�����һ�Σ�
C����Һʱ���������²�Һ���ϣ�
D������ʱ�������ɣ��������ȼ��ȣ�
��� �⣺A������ʱ���¶ȼƲⶨ��ֵ��¶ȣ���ʹ�¶ȼ�ˮ����������ƿ��֧�ܿڴ�����A��ȷ��
B����©ʱ��������ƿ��ע��������ˮ�����ϲ���ƿ����������ָ��סƿ�ף�����ʳָ��סƿ�������ã��۲��Ƿ�©ˮ����������ת180�㣬�ظ�������������B����
C����Һʱ���������²�Һ���ϣ����ȴ��¶˷ų��ܶȽϴ��Һ�壬�رջ��������Ͽڵ����ϲ�Һ�壬��C����
D������ʱ�������ɣ��������ȼ��ȣ�����ִ�������ʱֹͣ���ȣ���D����
��ѡA��
���� ���⿼�黯ѧʵ�鷽�������ۣ�Ϊ��Ƶ���㣬���ջ��������ᴿ��ʵ�鼼��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬ע��ʵ��������Է�������Ŀ�ѶȲ���


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
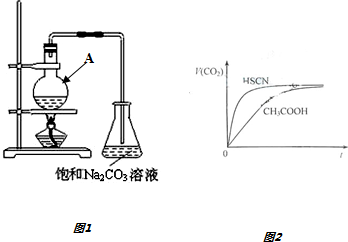
| ͬһ��Ӧʱ�� | ͬһ��Ӧ�¶� | ||||
| ��Ӧ�¶�/�� | ת���� ��%�� | ѡ���ԣ�%��* | ��Ӧʱ��/h | ת���ʣ�%�� | ѡ���� ��%��* |
| 40 | 77.8 | 100 | 2 | 80.2 | 100 |
| 60 | 92.3 | 100 | 3 | 87.8 | 100 |
| 80 | 92.6 | 100 | 4 | 92.3 | 100 |
| 120 | 94.5 | 98.7 | 6 | 93.0 | 100 |
| *ѡ����100%��ʾ��Ӧ���ɵIJ���������������ˮ | |||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5.04g | B�� | 3.6g | C�� | 6.48g | D�� | 3.24g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com