
ij���������ú����Ŀ�����A��ȡˮ����G��������ͼ��ʾ����֪��B����Ԫ�ص���������Ϊ30%������C���γ��������Ⱦ��֮һ��

(1) A����Ҫ�ɷֵĻ�ѧʽ����Ϊ ��ֻдһ�֣���
(2)����a������ ���ܡ����ܣ���F��Һֱ�����ɡ�
(3)ȷ��F��Һ�в�����Fe3���ķ�����___________ ___��
(4)��Ӧ���д��ڶ��ַ�Ӧ����д���������ڷ�������ԭ��Ӧ�����ӷ���ʽ
___ ___��
(5)��ҵ�Ϸ�ӦC��D�Ļ�ѧ����ʽ��_________ _____��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶ȣ��棩 | 20 | 40 | 60 | 80 | 100 |
| ʯ�� | 0.32 | 0.26 | 0.15 | 0.11 | 0.07 |
| ���� | 32 | 44.6 | 61.8 | 83.8 | 114 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���г����������ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
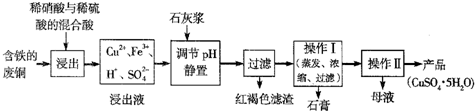
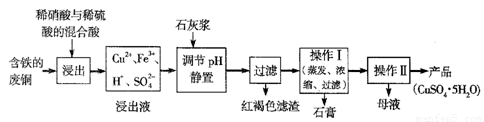
��12�֣�����ͭ��������������Ҫ�Ļ���ԭ�ϡ�
��1��������ij�����ú����ķ�ͭΪԭ������������CuSO4��5H2O������������ʾ��ͼ��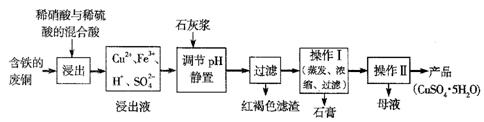
������ʯ���ڲ�ͬ�¶��µ��ܽ�ȣ���100gˮ�����±���
| �¶ȣ��棩 | 20 | 40 | 60 | 80 | 100 |
| ʯ�� | 0.32 | 0.26 | 0.15 | 0.11 | 0.07 |
| ���� | 32 | 44.6 | 61.8 | 83.8 | 1 14 14 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱���г����������ڶ���ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ������
��12�֣�����ͭ��������������Ҫ�Ļ���ԭ�ϡ�
��1��������ij�����ú����ķ�ͭΪԭ������������CuSO4��5H2O������������ʾ��ͼ��
������ʯ���ڲ�ͬ�¶��µ��ܽ�ȣ���100gˮ�����±���
|
�¶ȣ��棩 |
20 |
40 |
60 |
80 |
100 |
|
ʯ�� |
0.32 |
0.26 |
0.15 |
0.11 |
0.07 |
|
���� |
32 |
44.6 |
61.8 |
83.8 |
114 |
��ش��������⣺
�ٺ��ɫ��������Ҫ�ɷ���
��д��������������������ͭ�Ļ�ѧ����ʽ ��
�۲���I���¶�Ӧ�ÿ����� ���ң�
�ܴ���Һ�з��������ͭ����IJ�����ӦΪ��������Һ �� ��ϴ�ӡ����
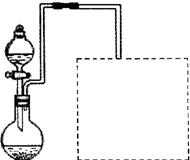
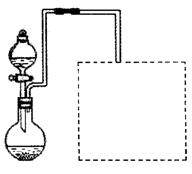
��2��ij��ȤС����ʵ���������ۺ�ϡ���ἰ��ͼװ����ȡFe��NO3��3���̶�����
�����ͼ���װ��δ��������
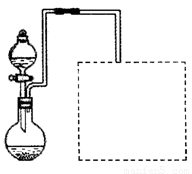
��Ϊ��֤ԭ�ϵij�����ã����ӿ췴Ӧ���ʣ������������¶ȿ�����100�����ڡ���ʵ���ж�Բ����ƿ���ȵ���ѷ�ʽ�� ���ȣ�
������ͼ�����߿��ڻ���β������װ�ã���ʾ����Һ�ɿ��١�������ղ����ĵ����������壩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2010�������ѧ����ĩ��ѧ������� ���ͣ������
ij���������ú����Ŀ�����A��ȡˮ����G��������ͼ��ʾ����֪��B����Ԫ�ص���������Ϊ30%������C���γ��������Ⱦ��֮һ��
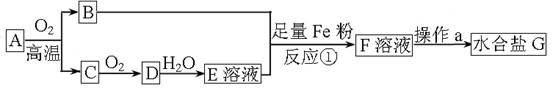
(1) A����Ҫ�ɷֵĻ�ѧʽ����Ϊ ��ֻдһ�֣���
(2)����a������ ���ܡ����ܣ���F��Һֱ�����ɡ�
(3)ȷ��F��Һ�в�����Fe3���ķ�����___________ ___��
(4)��Ӧ���д��ڶ��ַ�Ӧ����д���������ڷ�������ԭ��Ӧ�����ӷ���ʽ
___ ___��
(5)��ҵ�Ϸ�ӦC��D�Ļ�ѧ����ʽ��_________ _____��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com