
 Խ��
Խ��
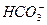 �� H�� Ka1��H2CO3��=4.45��10��7
�� H�� Ka1��H2CO3��=4.45��10��7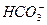

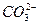 ��H�� Ka2(HCO3��)=5.61��10��11
��H�� Ka2(HCO3��)=5.61��10��11 H����
H����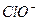 Ka(HClO)=2.95��10��8
Ka(HClO)=2.95��10��8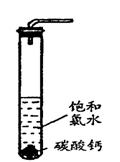
 �棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��
�棬�ܷ���ټ��ȡ������ƿ�е�Һ�壬ֱ����Ͳ�е����岻�����ӣ�������HClO�ķֽ⣩��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
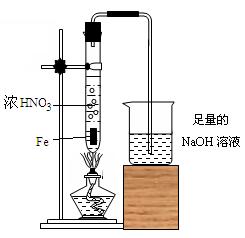
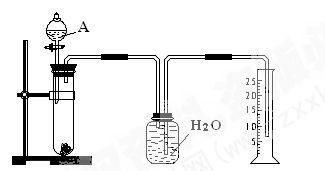
 ����ʳ����ȡŨHCl��ŨHCl����KMnO4��ȡC
����ʳ����ȡŨHCl��ŨHCl����KMnO4��ȡC l2��ѡ��
l2��ѡ�� ��ͼ��ʾװ�ã�������ʢ�ŵ��Լ������ʵ�顣
��ͼ��ʾװ�ã�������ʢ�ŵ��Լ������ʵ�顣
 HCl��Cl2�Ļ�ѧ��Ӧ����ʽ��
HCl��Cl2�Ļ�ѧ��Ӧ����ʽ�� _________________________________��
_________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣�
mol/L���ᡢ2mol/LNaOH��Һ��20%KSCN��Һ���������ʵ��̽�����̣� ����Ԥ������ͽ��ۡ�
����Ԥ������ͽ��ۡ�| ��� | ʵ����� | ʵ������ | ���� |
| �� | ��ҩ��ȡ������Ʒ�������Թ�A�У����õι�ȡ����  ____ ��Һ���μӵ��Թ�A�У���ַ�Ӧ ____ ��Һ���μӵ��Թ�A�У���ַ�Ӧ | �й���ʣ�࣬�������ݲ��� | �Ͻ��г��������Fe��Cu Ԫ�� |
| �� | ���Թ�A��ʣ������мӹ��� ________ ����ַ�Ӧ���ã�ȡ�ϲ���Һ���Թ�B�� | ���岿���ܽ⣬��������ų�����Һ��dz��ɫ | |
| �� | ���Թ�B�м������� _____���ٵμ�KSCN��Һ | _____ | |
| �� | ����ʣ������м���ϡ����ٵμ� ____________ ��Һ. | �����ܽ⣬����ɫ�̼�������������ܿ��ɺ���ɫ����Һ����ɫ���ټ�ij��Һ������ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
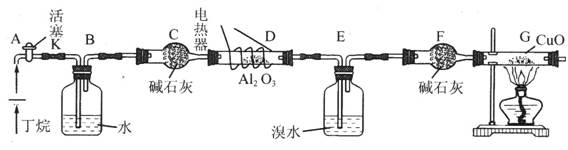 ע��CuO�ܽ���������CO2��H2O�������װ������ȥ��
ע��CuO�ܽ���������CO2��H2O�������װ������ȥ�� �������I��II�IJ������Ʒֱ��ǣ�I ,II ��
�������I��II�IJ������Ʒֱ��ǣ�I ,II ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ����� | ʵ������ | ʵ����� |
| A | ��ij��Һ�м���ϡ���ᣬ��������������ͨ�����ʯ��ˮ�� | ��ɫ��ζ������ʹ����ʯ��ˮ����� | ԭ��Һ�к���CO32? |
| B | ��������ͨ����ˮ�� | ��Һ��ɫ | ����������Ư���� |
| C | ���ۺ�ϡ�����Ϲ��Ⱥ��ټ���������������ͭ����Һ | ������ɫ���� | ����ˮ������������� |
| D | ȡ����ij��ɫ��Һ���ȵμ���ˮ���ټ����������Ȼ�̼�������� | ��Һ�ֲ㣬�²�ʳȺ�ɫ | ԭ��ɫ��Һ��һ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢݢ� | B���ۢܢޢ� | C���ڢۢݢ� | D���ڢܢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
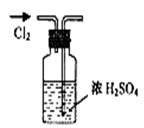
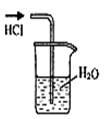
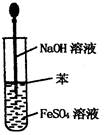
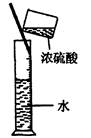
| A������Cl2 | B������NH3 | C����ȡFe(OH)2���� | D��ϡ��ŨH2SO4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com