
өӘСх»ҜОпКЗҙуЖшОЫИҫОпЦ®Т»Ј¬ПыіэөӘСх»ҜОпөД·Ҫ·ЁУР¶аЦЦЎЈ
ЈЁ1Ј©АыУГјЧНйҙЯ»Ҝ»№ФӯөӘСх»ҜОпЎЈТСЦӘЈә
ўЩCH4 (g)Ј«4NO2(g)ЈҪ4NO(g)Ј«CO2(g)Ј«2H2O(gЈ©?? ЎчH ЈҪЈӯ574 kJ/mol
ўЪCH4(g)Ј«4NO(gЈ©ЈҪ 2N2(g)Ј«CO2(g)Ј«2H2O(gЈ©? ЎчH ЈҪЈӯ1160 kJ/mol
ФтCH4 Ҫ«NO2 »№ФӯОӘN2 өДИИ»ҜС§·ҪіМКҪОӘЈә??????????? ???????????? ЎЈ
 ЈЁ2Ј©АыУГNH3ҙЯ»Ҝ»№ФӯөӘСх»ҜОпЈЁSCRјјКх)ЎЈёГјјКхКЗДҝЗ°УҰУГЧо№г·әөДСМЖшөӘСх»ҜОпНСіэјјКхЎЈ ·ҙУҰөД»ҜС§·ҪіМКҪОӘ:
ЈЁ2Ј©АыУГNH3ҙЯ»Ҝ»№ФӯөӘСх»ҜОпЈЁSCRјјКх)ЎЈёГјјКхКЗДҝЗ°УҰУГЧо№г·әөДСМЖшөӘСх»ҜОпНСіэјјКхЎЈ ·ҙУҰөД»ҜС§·ҪіМКҪОӘ: ОӘМбёЯөӘСх»ҜОпөДЧӘ»ҜВКҝЙІЙИЎөДҙлК©КЗ???????? ????????????????????????? ЈЁРҙіц1МхјҙҝЙЈ©ЎЈ
ОӘМбёЯөӘСх»ҜОпөДЧӘ»ҜВКҝЙІЙИЎөДҙлК©КЗ???????? ????????????????????????? ЈЁРҙіц1МхјҙҝЙЈ©ЎЈ
ЈЁ3Ј©АыУГClO2 Сх»ҜөӘСх»ҜОпЎЈЖдЧӘ»ҜБчіМИзПВ:? NO NO2
NO2 N2ЎЈТСЦӘ·ҙУҰўсөД»ҜС§·ҪіМКҪОӘ2NO+ ClO2 + H2O ЈҪNO2 + HNO3 + HClЈ¬Фт·ҙУҰўтөД»ҜС§·ҪіМКҪКЗ???????? ????? Ј»ИфЙъіЙ11.2 L N2ЈЁұкЧјЧҙҝцЈ©Ј¬ФтПыәДClO2 ??????? g ЎЈ
N2ЎЈТСЦӘ·ҙУҰўсөД»ҜС§·ҪіМКҪОӘ2NO+ ClO2 + H2O ЈҪNO2 + HNO3 + HClЈ¬Фт·ҙУҰўтөД»ҜС§·ҪіМКҪКЗ???????? ????? Ј»ИфЙъіЙ11.2 L N2ЈЁұкЧјЧҙҝцЈ©Ј¬ФтПыәДClO2 ??????? g ЎЈ
ЈЁ4Ј©УГ»оРФМҝ»№Фӯ·ЁҙҰАнөӘСх»ҜОпЈ®УР№Ш·ҙУҰОӘЈәCЈЁsЈ©+2NOЈЁgЈ© N2 ЈЁgЈ©+CO2 ЈЁgЈ©ЎчHЈ®ДіСРҫҝРЎЧйПтДіГЬұХИЭЖчјУИлТ»¶ЁБҝөД»оРФМҝәНNOЈ¬әгОВЈЁT1ЎжЈ©МхјюПВ·ҙУҰЈ¬·ҙУҰҪшРРөҪІ»Н¬КұјдІвөГёчОпЦКөДЕЁ¶ИИзПВЈә
N2 ЈЁgЈ©+CO2 ЈЁgЈ©ЎчHЈ®ДіСРҫҝРЎЧйПтДіГЬұХИЭЖчјУИлТ»¶ЁБҝөД»оРФМҝәНNOЈ¬әгОВЈЁT1ЎжЈ©МхјюПВ·ҙУҰЈ¬·ҙУҰҪшРРөҪІ»Н¬КұјдІвөГёчОпЦКөДЕЁ¶ИИзПВЈә
ЕЁ¶И/mol?L-1/ Кұјд/min | NO | N2 | CO2 |
0 | 0.100 | 0 | 0 |
10 | 0.058 | 0.021 | 0.021 |
20 | 0.040 | 0.030 | 0.030 |
30 | 0.040 | 0.030 | 0.030 |
40 | 0.032 | 0.034 | 0.017 |
50 | 0.032 | 0.034 | 0.017 |
ўЩT1ЎжКұЈ¬ёГ·ҙУҰөДЖҪәвіЈКэK= ????????? ЈЁұЈБфБҪО»РЎКэЈ©Ј®ўЪ30minәуЈ¬ёДұдДіТ»МхјюЈ¬·ҙУҰЦШРВҙпөҪЖҪәвЈ¬ФтёДұдөДМхјюҝЙДЬКЗ ????????????? Ј®ўЫИф30minәуЙэёЯОВ¶ИЦБT2ЎжЈ¬ҙпөҪЖҪәвКұЈ¬ИЭЖчЦРNOЎўN2ЎўCO2өДЕЁ¶ИЦ®ұИОӘ5Јә3Јә3Ј¬ФтёГ·ҙУҰөДЎчH ?? 0ЈЁМоЎ°ЈҫЎұЎўЎ°=Ўұ»тЎ°ЈјЎұЈ©Ј®
ЈЁ1Ј©CH4 (g)Ј«2NO2(g)ЈҪN2(g)Ј«CO2(g)Ј«2H2O(gЈ©?? ЎчH ЈҪЈӯ867 kJ/mol
ЈЁ2Ј©ФцҙуNH3өДЕЁ¶И»тјхРЎ·ҙУҰМеПөөДС№Зҝ»тҪөөН·ҙУҰМеПөөДОВ¶ИөИ
ЈЁ3Ј©2NO2 + 4 Na2SO3=N2 + 4 Na2SO4??? 67.5? ЈЁ4Ј©ўЩ0.56? ўЪ јхЙЩCO2ЕЁ¶И? ўЫ Јј
ЎҫҪвОцЎҝ
КФМв·ЦОцЈәЈЁ1Ј©ЈЁўЩЈ«ўЪЈ©ЎВ2ҝЙөГCH4 (g)Ј«2NO2(g)ЈҪN2(g)Ј«CO2(g)Ј«2H2O(gЈ©?? ЎчH ЈҪЈӯ867 kJ/molЎЈЈЁ2Ј©УЙУЪ·ҙУҰ өДХэ·ҙУҰЖшМеМе»эФцҙуөД·ЕИИ·ҙУҰЎЈЛщТФМбёЯөӘСх»ҜОпөДЧӘ»ҜВКҝЙІЙИЎөДҙлК©ҝЙТФКЗНЁ№эФцҙуNH3өДЕЁ¶И»тјхРЎ·ҙУҰМеПөөДС№Зҝ»тҪөөН·ҙУҰМеПөөДОВ¶ИөИҙлК©АҙКөК©ЎЈЈЁ3Ј©УЙМвДҝМṩөДРЕПўҝЙЦӘNO2УлNa2SO3·ўЙъСх»Ҝ»№Фӯ·ҙУҰЈә2NO2 + 4 Na2SO3 =N2 + 4 Na2SO4Ј¬ҙУ¶шПыіэБЛNOx¶Ф»·ҫіФміЙөДОЫИҫЎЈУЙ·ҪіМКҪөГ№ШПөКҪЈә2ClO2Ў«2NO2 Ў«N2ЎЈn(N2)=0.5mol,ЛщТФn(ClO2)=1molЈ¬m(ClO2)= 1molЎБ67.5g/mol=67.5gЎЈЈЁ4Ј©ўЩT1ЎжКұЈ¬ёГ·ҙУҰөДЖҪәвіЈКэ
өДХэ·ҙУҰЖшМеМе»эФцҙуөД·ЕИИ·ҙУҰЎЈЛщТФМбёЯөӘСх»ҜОпөДЧӘ»ҜВКҝЙІЙИЎөДҙлК©ҝЙТФКЗНЁ№эФцҙуNH3өДЕЁ¶И»тјхРЎ·ҙУҰМеПөөДС№Зҝ»тҪөөН·ҙУҰМеПөөДОВ¶ИөИҙлК©АҙКөК©ЎЈЈЁ3Ј©УЙМвДҝМṩөДРЕПўҝЙЦӘNO2УлNa2SO3·ўЙъСх»Ҝ»№Фӯ·ҙУҰЈә2NO2 + 4 Na2SO3 =N2 + 4 Na2SO4Ј¬ҙУ¶шПыіэБЛNOx¶Ф»·ҫіФміЙөДОЫИҫЎЈУЙ·ҪіМКҪөГ№ШПөКҪЈә2ClO2Ў«2NO2 Ў«N2ЎЈn(N2)=0.5mol,ЛщТФn(ClO2)=1molЈ¬m(ClO2)= 1molЎБ67.5g/mol=67.5gЎЈЈЁ4Ј©ўЩT1ЎжКұЈ¬ёГ·ҙУҰөДЖҪәвіЈКэ .ўЪУЙұнЦРКэҫЭҝЙТФҝҙіц30minәуЈ¬c(CO2)јхРЎЈ¬c(N2)ИҙЦрҪҘФцјУc(NO)УРЛщјхЙЩЎЈЛөГчёДұдөДМхјюКЗјхРЎc(CO2)ЎЈўЫИф30minәуЙэёЯОВ¶ИЦБT2ЎжЈ¬ҙпөҪЖҪәвКұЈ¬ИЭЖчЦРNOЎўN2ЎўCO2өДЕЁ¶ИЦ®ұИОӘ5Јә3Јә3Ј¬
.ўЪУЙұнЦРКэҫЭҝЙТФҝҙіц30minәуЈ¬c(CO2)јхРЎЈ¬c(N2)ИҙЦрҪҘФцјУc(NO)УРЛщјхЙЩЎЈЛөГчёДұдөДМхјюКЗјхРЎc(CO2)ЎЈўЫИф30minәуЙэёЯОВ¶ИЦБT2ЎжЈ¬ҙпөҪЖҪәвКұЈ¬ИЭЖчЦРNOЎўN2ЎўCO2өДЕЁ¶ИЦ®ұИОӘ5Јә3Јә3Ј¬ <0.56ЎЈЛөГчЙэёЯОВ¶ИЈ¬»ҜС§ЖҪәвПтДж·ҙУҰ·ҪПтТЖ¶ҜЎЈёщҫЭЖҪәвТЖ¶ҜФӯАн:ЙэёЯОВ¶ИЈ¬»ҜС§ЖҪәвПтОьИИ·ҙУҰ·ҪПтТЖ¶ҜЎЈДж·ҙУҰ·ҪПтОӘОьИИ·ҙУҰЈ¬ЛщТФёГ·ҙУҰөДХэ·ҙУҰОӘ·ЕИИ·ҙУҰЎЈФтёГ·ҙУҰөДЎчH Јј0Ј®
<0.56ЎЈЛөГчЙэёЯОВ¶ИЈ¬»ҜС§ЖҪәвПтДж·ҙУҰ·ҪПтТЖ¶ҜЎЈёщҫЭЖҪәвТЖ¶ҜФӯАн:ЙэёЯОВ¶ИЈ¬»ҜС§ЖҪәвПтОьИИ·ҙУҰ·ҪПтТЖ¶ҜЎЈДж·ҙУҰ·ҪПтОӘОьИИ·ҙУҰЈ¬ЛщТФёГ·ҙУҰөДХэ·ҙУҰОӘ·ЕИИ·ҙУҰЎЈФтёГ·ҙУҰөДЎчH Јј0Ј®
ҝјөгЈәҝјІй»ҜС§·ҪіМКҪЎўИИ»ҜС§·ҪіМКҪөДКйРҙЎў»ҜС§ЖҪәвіЈКэөДјЖЛгЎўНвҪзМхјю¶Ф»ҜС§ЖҪәвТЖ¶ҜөДУ°ПмЎЈ



| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈәФД¶БАнҪв
| V(іфСхҝХЖш) | V(СМЖш) |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈәФД¶БАнҪв
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
 өӘСх»ҜОпКЗҙуЖшОЫИҫОпЦ®Т»Ј¬ПыіэөӘСх»ҜОпөД·Ҫ·ЁУР¶аЦЦЈ®
өӘСх»ҜОпКЗҙуЖшОЫИҫОпЦ®Т»Ј¬ПыіэөӘСх»ҜОпөД·Ҫ·ЁУР¶аЦЦЈ®| 180Ўж |
| ҙЯ»ҜјБ |
| ClO2 |
| ·ҙУҰўс |
| Na2SO3 |
| ·ҙУҰўт |
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә2013-2014С§ДкәюДПКЎ»і»ҜКРёЯИэөЪТ»ҙОДЈДвҝјКФАнЧЫ»ҜС§КФҫнЈЁҪвОц°жЈ© МвРНЈәМоҝХМв
өӘСх»ҜОпКЗҙуЖшОЫИҫОпЦ®Т»Ј¬ПыіэөӘСх»ҜОпөД·Ҫ·ЁУР¶аЦЦЎЈ
ЈЁ1Ј©АыУГјЧНйҙЯ»Ҝ»№ФӯөӘСх»ҜОпЎЈТСЦӘЈә
CH4 (g)Ј«4NO2(g)ЈҪ4NO(g)Ј«CO2(g)Ј«2H2O(g)??? ЎчH ЈҪЈӯ574 kJ/mol
CH4(g)Ј«4NO(g) ЈҪ 2N2(g)Ј«CO2(g)Ј«2H2O(g)?? ЎчH ЈҪЈӯ1160 kJ/mol
ФтCH4 Ҫ«NO2 »№ФӯОӘN2 өДИИ»ҜС§·ҪіМКҪОӘ??????????????????????????????? ЎЈ???????
 ЈЁ2Ј©АыУГNH3ҙЯ»Ҝ»№ФӯөӘСх»ҜОпЈЁSCRјјКх)ЎЈёГјјКхКЗДҝЗ°УҰУГЧо№г·әөДСМЖшөӘСх»ҜОпНСіэјјКхЎЈ ·ҙУҰөД»ҜС§·ҪіМКҪОӘЈә2NH3(g)Ј«NO(g)Ј«NO2(g)
ЈЁ2Ј©АыУГNH3ҙЯ»Ҝ»№ФӯөӘСх»ҜОпЈЁSCRјјКх)ЎЈёГјјКхКЗДҝЗ°УҰУГЧо№г·әөДСМЖшөӘСх»ҜОпНСіэјјКхЎЈ ·ҙУҰөД»ҜС§·ҪіМКҪОӘЈә2NH3(g)Ј«NO(g)Ј«NO2(g)  ? 2N2(g)Ј«3H2O(g)ЎЎҰӨH < 0
? 2N2(g)Ј«3H2O(g)ЎЎҰӨH < 0
ОӘМбёЯөӘСх»ҜОпөДЧӘ»ҜВКҝЙІЙИЎөДҙлК©КЗ????????????????? ЈЁРҙіц1МхјҙҝЙЈ©ЎЈ
ЈЁ3Ј©АыУГClO2 Сх»ҜөӘСх»ҜОпЎЈЖдЧӘ»ҜБчіМИзПВЈә
NO NO2
NO2 N2
N2
ТСЦӘ·ҙУҰўсөД»ҜС§·ҪіМКҪОӘ2NO+ ClO2 + H2O ЈҪ NO2 + HNO3 + HClЈ¬Фт·ҙУҰўтөД»ҜС§·ҪіМКҪКЗ?? ????????????? Ј»ИфЙъіЙ11.2 L N2ЈЁұкЧјЧҙҝцЈ©Ј¬ФтПыәДClO2 ??????? g ЎЈ
ЈЁ4Ј©АыУГCOҙЯ»Ҝ»№ФӯөӘСх»ҜОпТІҝЙТФҙпөҪПыіэОЫИҫөДДҝөДЎЈ
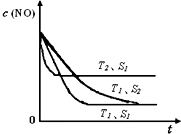
ТСЦӘЦКБҝТ»¶ЁКұЈ¬Фцҙу№ММеҙЯ»ҜјБөДұнГж»эҝЙМбёЯ»ҜС§·ҙУҰЛЩВКЎЈИзНјКЗ·ҙУҰ2NO(g) + 2CO(g)  2CO2(g)+ N2(g) ЦРNOөДЕЁ¶ИЛжОВ¶И(T)ЎўөИЦКБҝҙЯ»ҜјБұнГж»э(S)әНКұјд(t)өДұд»ҜЗъПЯЎЈҫЭҙЛЕР¶ПёГ·ҙУҰөДЎчH????? 0 (МоЎ°ЈҫЎұЎўЎ°ЈјЎұ»тЎ°ОЮ·ЁИ·¶ЁЎұ)Ј»ҙЯ»ҜјБұнГж»эS1????? S2 (МоЎ°ЈҫЎұЎўЎ°ЈјЎұ»тЎ°ОЮ·ЁИ·¶ЁЎұ)ЎЈ
2CO2(g)+ N2(g) ЦРNOөДЕЁ¶ИЛжОВ¶И(T)ЎўөИЦКБҝҙЯ»ҜјБұнГж»э(S)әНКұјд(t)өДұд»ҜЗъПЯЎЈҫЭҙЛЕР¶ПёГ·ҙУҰөДЎчH????? 0 (МоЎ°ЈҫЎұЎўЎ°ЈјЎұ»тЎ°ОЮ·ЁИ·¶ЁЎұ)Ј»ҙЯ»ҜјБұнГж»эS1????? S2 (МоЎ°ЈҫЎұЎўЎ°ЈјЎұ»тЎ°ОЮ·ЁИ·¶ЁЎұ)ЎЈ

Ійҝҙҙр°ёәНҪвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com