
A��B��C��D��E���Ƕ�����Ԫ�أ�ԭ��������������A��B����ͬһ���ڣ�C��D��Eͬ����һ���ڡ�C��B�ɰ�ԭ�Ӹ�����2��l��1��1�ֱ��γ��������ӻ�������ҡ�D��A��ԭ�Ӹ�����3��2�γ����ӻ��������E�ǵؿ��к�����ߵĽ���Ԫ�ء�����������Ϣ�ش��������⣺��ÿ��2�֣���8�֣�
��1��EԪ�������ڱ��е�λ����____________��
��2������A��ԭ�ӽṹ��ͼ
��3�����ӻ������ҵĵ���ʽ��
��4��C��D��E�γɵļ����Ӱ뾶�ɴ�С��˳���ǣ������ӷ��ű�ʾ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
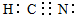
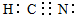
 CH3COOH+OH-
CH3COOH+OH- CH3COOH+OH-
CH3COOH+OH-�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��֪A��B��C��D��E����Ԫ�����ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��Bԭ�ӵ�p�����������γɵ��⻯��ķе���ͬ����Ԫ�ص��⻯������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A+��Dԭ���γɵ�������һ�����Ӳ㣮C��A�γ�A2C�����ӻ����E��ԭ������Ϊ26��Eԭ�ӻ�������Χ�н϶���������Ŀչ��������һЩ���ӻ������γ��������������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��
ʾ��λ�ڸ�������Ķ�������ģ��û�����Ļ�ѧʽ��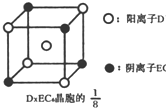
| Ԫ�� | Mn | Fe | |
| ���� ��/kJ?mol-1 |
��1 | 717 | 759 |
| ��2 | 1509 | 1561 | |
| ��3 | 3248 | 2957 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
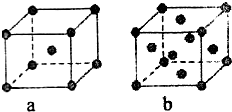 ��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA��C��B��D��E����֪Aԭ�ӵ�p���Ϊ����������γɵ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵģ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B+���ӱ�Dԭ���γɵ�������һ�����Ӳ㣮C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29��
��֪A��B��C��D��E����Ԫ�����ڱ��е�ǰ������Ԫ�أ�����ԭ�������Ĵ�С��ϵΪA��C��B��D��E����֪Aԭ�ӵ�p���Ϊ����������γɵ��⻯��ķе���ͬ����ǽ���Ԫ�ص��⻯������ߵģ�Dԭ�ӵõ�һ�����Ӻ���3p�����ȫ������B+���ӱ�Dԭ���γɵ�������һ�����Ӳ㣮C��B���γ�BC�͵����ӻ����E��ԭ������Ϊ29���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com