
����Ŀ������ȫ������Ϲ����к���ƻ����(MLA)�������ʽΪC4H6O5��0.1 molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48 L CO2(��״��)��ƻ������ˮ������ʹ��ˮ��ɫ�IJ��ƻ���ᾭ�ۺ����ɾ�ƻ����(PMLA)��
(1)д���������ʵĽṹ��ʽ��A____________________��D____________________��
(2)ָ����Ӧ���ͣ���__________����__________��
(3)д��������MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ��__________________��
(4)д��E��Fת���Ļ�ѧ����ʽ________________________��
(5)����ת����ϵ�в�����������˳���ܷ�ߵ���_______(��ܡ����ܡ�)˵�����ɣ�_________________________��
(6)PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ___________________��
���𰸡�
(1)CH2BrCH=CHCH2Br��OHCCH2CHBrCHO��
(2)�ӳɣ�ȡ����
(3)
(4)HOOCCH2CHBrCOOH+3NaOH��NaOOCCH2CH(OH)COONa+NaBr+2H2O��
(5)���ܣ�����������B��̼̼˫��Ҳ��������
(6)![]() ��
��![]() ��
��
��������
���������ƻ�������ʽΪC4H6O5��0��l molƻ����������NaHCO3��Һ��Ӧ�ܲ���4.48L CO2(��״��)��������̼�����ʵ���Ϊ0.2mol����1molƻ���Ậ2mol-COOH��ƻ������ˮ������ʹ��ˮ��ɫ�IJ��Ӧ����1��-OH�����ƻ����ķ���ʽ֪��ƻ����Ľṹ��ʽΪ��HOOCCH2CH(OH)COOH��ƻ����������Ӧ���еľۺ����ɾ�ƻ����(PMLA)����ṹΪ![]() ��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH(OH)COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ����(PMLA)��
��D����������E����E�к�����ԭ�ӣ�E���������Ƶ�ˮ��Һ������Ӧ����F��F�ữ����MLA������F�Ľṹ��ʽΪ��NaOOCCH2CH(OH)COONa��E�Ľṹ��ʽΪ��HOOCCH2CHBrCOOH��D�ܷ���������Ӧ��D�к���ȩ��������D�Ľṹ��ʽΪ��OHCCH2CHBrCHO������1��3-����ϩ��D�Ľṹ��ʽ֪��1��3-����ϩ���巢��1��4�ӳ�����AΪBrCH2CH=CHCH2Br��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����BΪHOCH2CH=CHCH2OH��B��HBr�����ӳɷ�Ӧ����C��C�Ľṹ��ʽΪ��HOCH2CH2CHBrCH2OH��C�ٱ���������D��ƻ���ᾭ�ۺ����ɾ�ƻ����(PMLA)��
(1)������������֪��AΪCH2BrCH=CHCH2Br��DΪOHCCH2CHBrCHO���ʴ�Ϊ��CH2BrCH=CHCH2Br��OHCCH2CHBrCHO��
(2)��Ӧ����1��3-����ϩ���巢��1��4�ӳ�����BrCH2CH=CHCH2Br����Ӧ����BrCH2CH=CHCH2Br���������Ƶ�ˮ��Һ����ȡ����Ӧ����HOCH2CH=CHCH2OH���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��
(3)������MLA������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ�У�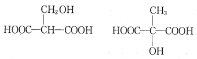 ���ʴ�Ϊ��
���ʴ�Ϊ��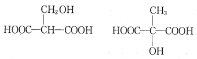 ��
��
(4)E��Fת���Ļ�ѧ����ʽΪ��HOOCCH2CHBrCOOH+3NaOH��NaOOCCH2CH(OH)COONa+NaBr+2H2O���ʴ�Ϊ��HOOCCH2CHBrCOOH+3NaOH��NaOOCCH2CH(OH)COONa+NaBr+2H2O��
(5)˳���ܵߵ�������������B��̼̼˫��Ҳ���������ʴ�Ϊ�����ܣ�����������B��̼̼˫��Ҳ��������
(6)PMLA�������õ����������ԣ�������Ϊ��������ߵȲ���Ӧ��������ҽҩ�������������������������ˮ��Ļ�ѧ����ʽΪ��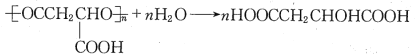 ���ʴ�Ϊ��
���ʴ�Ϊ��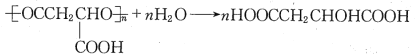 ��
��


 Ӣ�żƻ���ĩ����ϵ�д�
Ӣ�żƻ���ĩ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ�����������������Ҫ��;���������ܼ�����������Ҳ��������ɫ�̶�Һ�������ܼ���ʳƷ��������л��ϳ��м��塣����ϩΪԭ���Ʊ�������������ĺϳ�·�����£�
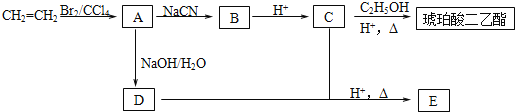
��֪����RBr+NaCN![]() RCN+NaBr
RCN+NaBr
��RCN+2H2O+H+![]() RCOOH+NH4+
RCOOH+NH4+
��ش��������⣺
��1����ϩ����A�ķ�Ӧ����Ϊ ��A�������� ��
��2��B�Ľṹ��ʽΪ ��������������Ľṹ��ʽΪ ��
��3��A![]() D�Ļ�ѧ����ʽΪ ��
D�Ļ�ѧ����ʽΪ ��
��4��EΪ��Ԫ��״�����E�к��еĹ���������Ϊ ��
��5���ܷ���������Ӧ��������̼���Ʒ�Ӧ����CO2��C��ͬ���칹���� �֡�
��6����������������������ĺϳ�·�ߣ����һ���ɱ���(CH3CH2CH2OH)�ϳ�2-������![]() �ĺϳ�·�ߣ� ��
�ĺϳ�·�ߣ� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ����ʵ��������Ҳ������ͼ��ʾ��װ����ȡ����������
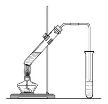
�ش��������⣺
��1���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ� ��
��2��Ũ����������ǣ��� ��
�� ��
��3������̼������Һ����Ҫ������ ��
��4��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У���Ŀ��
�� ��
��5����Ҫ���Ƶõ������������������Ӧ���õ�ʵ������� ��
��6������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ����ȩ������ͭ���£����Ա��������������ᡣ���ݴ�ԭ�������֤ʵ������ͼ��ʾ���Թ�A��װ��40������ȩˮ��Һ������ͭ��ĩ���Թ�C��װ����������ˮ�����ռ�����������Һ���ձ�B��װ��ijҺ��������֪��60�桫80��ʱ����˫�����������������ɷ�����ȩ��������Ӧ����������ʮ���η�Ӧ������ȫ���й����ʵķе���±���

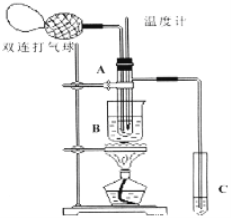
��ش��������⣺
��1���Թ�A����60��80��ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ������ ��
��2����ͼ��ʾ��ʵ��IJ�ͬ�Σ���Ҫ�����¶ȼ����Թ�A�ڵ�λ�ã���ʵ�鿪ʼʱ�¶ȼ�ˮ�����λ��Ӧ�� ��Ŀ���� �����Թ�A�ڵ���Ҫ��Ӧ��ɺ��¶ȼ�ˮ�����λ��Ӧ�� ��Ŀ���� ��
��3���ձ�B�������� ���ձ�B��ʢװ��Һ������� �� �����ϱ���������ѡ����д�ṹ��ʽ����
��4����������Թ�C���Ƿ��в������ᣬ����������ҩƷ�н���ѡ�����һ������ʵ�鷽�����ɹ�ѡ���ҩƷ�У�pH��ֽ����ɫ��ʯ����ֽ����ɫ�Ĵ���Ǧ��ֽ��̼�����Ʒ�ĩ��ʵ��������ѡ���÷���Ϊ________ ��
��5����֪��ȩ�ܱ���ˮ������д���÷�Ӧ�Ļ�ѧ����ʽ______________________________ .
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ���㾫��![]() ����ʳƷ��ҩƷ����ױƷ�ȷ���Ӧ�ù㷺��������A�ϳ��㾫�����������л����·�����¡�
����ʳƷ��ҩƷ����ױƷ�ȷ���Ӧ�ù㷺��������A�ϳ��㾫�����������л����·�����¡�
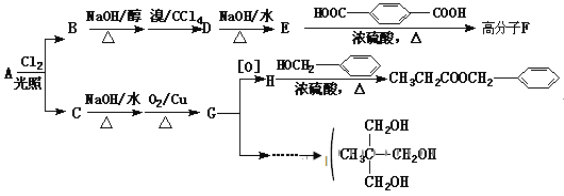
��֪��A��������һ�ȴ���B��C��
�ش��������⣺
��1����![]() ��Ϊͬ���칹�����____________________ �����������
��Ϊͬ���칹�����____________________ �����������
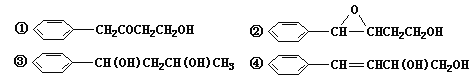
��2��D���ʵ�����Ϊ______________________________��
��3���߷���F�Ľṹ��ʽΪ______________________________��
��4��д��H��I��Ӧ���������Ļ�ѧ����ʽ��_________ ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ��ij��ȤС��ͬѧ��ʵ�����ü���l��������ŨH2SO4���廯�ƻ����ķ������Ʊ�1���嶡�飬���������ͼ��ʾ��ʵ��װ�������еļг�����û�л�������

��ش��������⣺
��1��Aװ���У����ձ����Һ�浹��һ��©������Ŀ���� ������װ���ж��õ��������ܣ�Aװ������ˮ�� ������ĸ���������룬Bװ������ˮ�� ������ĸ���������롣
��2���Ʊ������У������Ũ�������ȱ������ϡ�ͣ���Ŀ���� ��������ĸ��
a�����ٸ�����ϩ���ѵ����� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
��3����ͬѧ��ͨ����������Ǽ������ò������Ƿ��С���CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ�����CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���� ��
��4��Ϊ�˽�һ���ᴿ1���嶡�飬��С��ͬѧ�������л�����й��������±���
���� | �۵㣯�� | �е㣯�� |
1������ | ��89.5 | 117.3 |
1���嶡�� | ��112.4 | 101.6 |
���� | ��95.3 | 142.4 |
1����ϩ | ��185.3 | ��6.5 |
����Bװ����ɴ��ᴿʵ��ʱ��ʵ����ҪѸ�������¶��� �ռ�������֡�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ���л���G�Ǻϳ�ij�����Ⱦۺ���ĵ��壬��ϳɷ������£�

��֪��R1COOR2+R3OH![]() RCOOR3+R2OH
RCOOR3+R2OH
(1)����ϵͳ��������A������Ϊ____________��
(2)���������е�ȡ����Ӧ���ڡ������__________________(�����)
(3)������E�ĺ˴Ź�������ͼ�й��ж�����շ壬����ķ������Ϊ________��
(4)����ͼ�ϳ�·�߿ɵõ�F��ͬ���칹��H����ɼ���H��F���Լ���_____________

(5)C������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ______________ ��
(6)J��C��Ϊͬ���칹�壬������������ʡ�����Ҫ����л�����_______�֣������������칹��
�� ����NaOH��Һ��Ӧ����1mol J��ȫ��Ӧ����4molNaOH��
�� �ܷ���������Ӧ����1mol J��������������Һ��Ӧ����4molAg��
�� �����б����ϵ�һ��ȡ����ֻ��һ�֡�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ���л���G��һ��ҽҩ�м��壬��ͨ����ͼ��ʾ·�ߺϳɡ�A��ʯ�ͻ�������Ҫ��Ʒ�ҷ���������ԭ����ͬһƽ���ϣ�H�ķ���ʽ��C7H8��[
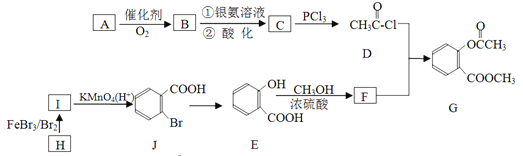
��֪��![]()
��ش��������⣺
��1��A������ʽ��________________________________��
��2��C��D�ķ�Ӧ������________________________________��
��3����һ��������������E���ӷ������Ӽ���ˮ����һ�ֻ�״����д���û�״�����ṹ��ʽ__________��
��4��G������������������Һ��Ӧ�Ļ�ѧ����ʽ��________________________________��
��5����������������F��ͬ���칹��(����F)����_________________����
�������Ȼ�����Һ������ɫ��Ӧ�ڿ��Է���ˮ�ⷴӦ
���к˴Ź����������շ����ٵ�ͬ���칹��Ľṹ��ʽΪ___________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ������ᱽ������C18H16O2���㷺�����㾫�ĵ������Ϊ�˺ϳɸ����ʡ�ijʵ���ҵĿƼ���Ա��������кϳ�·�ߣ�

�Իش��������⣺
��1��A�Ľṹ��ʽΪ____________��F�Ľṹ��ʽΪ____________��
��2����Ӧ�������ӵ��Լ���____________����Ӧ�ݵ�һ��������____________��
��3����Ӧ�۵Ļ�ѧ����ʽΪ___________________________________��
��4�������ϳ�·��������ȡ����Ӧ����____________ ����������
��5��C��ͬ���칹���У�ֻ��һ�������ŵķ�����_______�֣�д���������������ҿ��Է���������Ӧ�����з��ӵĽṹ��ʽ__________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com