
������ˮ�Ͷ�����̼������������Ҫ�����ʡ�
��1��A��B��C�����о�������ɺ����ʵ�ʵ�顣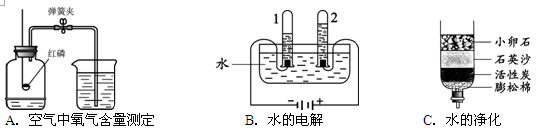
�ٹ���Aͼ��ʾʵ�飬����˵������ȷ���� ��
A��ʵ��ʱ����Ӧ���� B����ȼ����ǰ���õ��ɼмн��齺��
C������Ϩ������̴��ɼ� D�����ս���ƿ��ˮ�����ԼΪ���������
��Bͼ�Թ�1�е�����Ϊ ��Cͼ�о���ˮ�ķ����� ��������
��2����ʯ�ҽ�Ĩǽ��һ��ʱ���ǽ�ڱ���ְ���Ӳ��ԭ���� ���û�ѧ����ʽ�ش𣩡�
��3��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�¶�����̼�Ͱ�����NH3�����Ժϳ�����[CO(NH2)2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��4����̼�����Ե��ܺġ����ŷš�����ȾΪ��������ʵ���������Դ����Ч�ʺʹ��������Դ�ṹ������˵���У���ȷ���� ��
A����������ʱ����ú������ú����ʹ��
B����У��������͵��������շ��糧�����շ���
C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ�
D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դx
��1����C ��H2 ����
��2��Ca(OH)2+CO2=CaCO3��+H2O
��3��CO2+2NH3 H2O+CO(NH2)2
H2O+CO(NH2)2
��4��ABCD
���������������1���ٸ�ʵ���Dzⶨ����������������ʵ�顣��ʵ���ԭ�����������ʽ�������Ӧ�������������ѹ���ͣ�����ˮ��������Dz��뷴Ӧ�������������ʵ��ɹ����Ĺؼ��У�1������Ҫ������2��װ��������Ҫ���ã�3������ʱ�¶�Ҫ�������¡�����C�������ɶ���ƫ�ͣ���
��BͼΪ���ˮʵ�顣�Թ�1�е�����������Թ�Ҫ�࣬���Թ�1���Դ�ĸ������������Բ���������Ϊ�������Թ�2�ڵ�����Ϊ������
Cͼ������С��ʯ��ʯӢɳ�����ʶ�ˮ�����˹��˾��������û���̿��ˮ����������������
��2��ʯ�ҽ��ijɷ����������ƣ�����������еĶ�����̼��Ӧ����ѧ����ʽΪ��Ca(OH)2+CO2=CaCO3��+H2O
��3�������п�֪����Ӧ��Ϊ�����Ͷ�����̼������Ϊ���¸�ѹ��������Ϊ���غ�ˮ�����Է�Ӧ�Ļ�ѧ����ʽΪ��CO2+2NH3 H2O+CO(NH2)2
H2O+CO(NH2)2
��4��A����������ʱ����ú������ú����ʹ�ÿ����ӿ�ȼ���������ĽӴ�������Ӷ��ﵽ���ȼ�������ʵ����á�����Ҫ��B����У��������͵��������շ��糧�����շ��硱�ȼ�С����Щ������������Ⱦ�����γ�һ���ĵ��ܡ�����Ҫ��C���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ硱�����˶�̫���ܵ����ã������˶Ե��ܵ�������Ҫ��D������̫���ܵ������Դ���滯ʯȼ�ϣ������ڽ�Լ��Դ�������˶�̫���ܵ����á�����Ҫ��
���㣺���������ʡ���ѧ����ʽ����д����ɫ��ѧ



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�ˮ��һ����������������ģ�����Ӧ���˽��й�ˮ��һЩ֪ʶ��
��1������ˮ�������䰮ˮ��Դ����ÿ������Ӧ�������κ������������������ڱ���ˮ��Դ���� (�����)��
A������ʹ�û���ũҩ B����ҵ��ˮ���������ŷ�
C��ʹ�ú���ϴ�·� D��������ˮֱ���ŷ�
��2��ˮҲ��������ܼ������������ʼ���������ˮ�У����γ���ɫ��Һ���� ��������ĸ��ţ�
A��ֲ���� B������ C��CaCO3 D���������
��3���ܽ��˽϶�ĵĿ����Ըƺ�þ�Ļ������ˮ����Ӳˮ��Ӳˮ������������в���Ӱ�졣�����У�һ����� �ķ�����ʹӲˮת��Ϊ��ˮ��
��4���ҹ����Ƴ���Ư�۸���Ч������ˮ��������ClO2������ȡClO2��ӦΪ��
X + 2NaClO2 ="=" 2ClO2 + 2NaCl����X�Ļ�ѧʽΪ ��
��5��д��һ����ˮ�μӵĻ��Ϸ�Ӧ�Ļ�ѧ��Ӧ����ʽ�� ��
��6����ʵ������Ũ��������ϡ����ʱ����Ҫ�õ�ˮ������Ҫ�����У����㡢 �����ȡ���ȴ������װƿ�����ϱ�ǩ�� ��Ҫ����184g��������Ϊ10%��ϡ���ᣬ��Ҫ��������Ϊ98%��Ũ����(�ܶ�Ϊ1.84 g/cm3) mL(����������һλС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��д����ѧ����ʽ���ش��й����⣺
��1��ˮ��ͨ��ʱ������⣺��_ ________����
�ڸ�ʵ���У��ɿ��������븺������������������_________������ʵ��ó��Ľ�����ˮ����____ _____��ɡ�
��2��ϸ��˿�ڴ�������ȼ�գ���__ _______����
��ʵ����Ҫ����ƿ��Ԥ�ȷ�������ˮ����ϸɳ��ԭ������___ __ ____ ����
��3����ͼ��ͼ�к���ȼ�յĻ�ѧ����ʽΪ��_______ __ ����
��ֹˮ�к�����������_______ __��˵��������������Լռ_ ___������ͨ��ʵ��ó�������۵Ļ�ѧ����__ __������ţ���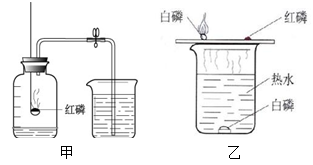
| A�������� | B�������� | C�������� | D����ķ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijС��ͬѧΪ̽�����ӵ���������������������ʵ�飬����������У����ش��������⣺
̽��ʵ��1����λͬѧ����100mL��Ͳ�ֱ�ȡ50mLˮ��50mL�ƾ���Ȼ��������Һ��������һ֧��Ͳ�л�ϣ�Ƭ�̺�����������___________������ڡ��������ڡ���С�ڡ���100mL����Ľ���_________________________________��
̽��ʵ��2��ijͬѧΪ�Ƚ�Ʒ���������ˮ����ˮ����ɢ�����ʲ�ͬ������˶���ʵ�飬��ʵ���г�ѡ����ͬ�������ձ���ͬʱ����Ʒ���⣬����Ϊ����Ҫ���Ƶı�����______________________���۲������_________________________________��
ʵ��̽��3��С��ͬѧ������ͼʵ��̽��ˮ������ֱ���������µı仯��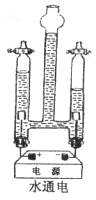
���ô�����ľ���ӽ�Һ���½����ٵIJ����ܼ��촦�������������۲쵽��������______________________��
����ȼ��ľ���ӽ�Һ���½��϶�IJ����ܼ��촦�������������۲쵽��������______________________��
��д��ˮͨ�緢���仯�����ֱ���ʽ______________________��
�ܸ�ʵ�������ϲ������������������֮��ӦΪ2��1����ʵ�ʹ۲쵽���������������֮���Դ���2��1����IJ�����______________________��
�ݸ�ʵ��֤ʵ�˷��ӵ�������_________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����벻��ˮ
��1�������У�����Ӳˮ����ˮ�� ��������������ˮ���м����Ҷ�����Ŀ���� ��
��2��ʵ���ҵ��ˮ����Դ���������������� ���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ������ͨ�����������֮һ��
��1�������еġ�ˮ���кܶ��֡����С�ˮ�����ڴ�������� (����ĸ���)��
| A����ˮ | B������ˮ | C������ˮ | D����Ȫˮ |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ĿǰΪ�ֿ����ϴ���������֯��������������ȡ����ˮ�������նɹ��ѹأ��������Ⱦ��Ӳ�ȴ�ĵ���ˮ���������彡����Ϊ�����ǻ�Ӧ������н�һ���Ĵ�����
��1���ⶨ����ˮ�����ȣ��������� ����
��2������ˮ������������������������������ͨ��ʹ��Ư�ۣ�����Ч�ɷִ�����ƿɷ������·�Ӧ��Ca��ClO��2+X+H2O=CaCO3��+2HClO����X�Ļ�ѧʽΪ�� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д����Ӧ�����ֱ���ʽ��ע��������Ӧ����
��1����˿��������ȼ��________________________________________ ���� ��
��2)��������غͶ������̵Ļ����������___________________________________ ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ�����ǵ�����������ϢϢ��أ�����ش�
��1����Ȼˮ�к����������ʣ����������������������˺�����ȷ����������������ڹ���ʱ����������� ����ˮ�����г����� ����ˮ�е���ɫ����ζ��
��2��Ӳˮ����������������ܶ��鷳�������пɲ��ý���ˮ��Ӳ�ȵķ����� ��
��3������ˮ��Դ��ÿ����������κ��������о�һ�ֽ�Լ��ˮ�ľ����ʩ
��4��������۲�ͼ��A��B��װ�ã�д��Bװ���з�����Ӧ�Ļ�ѧ����ʽ ��ʵ��˵��ˮ���� ��ɵģ��÷��Ӻ�ԭ�ӵĹ۵�����Ƚ�Aװ�ú�Bװ����ʵ�������ˮ�ı仯�����A��ˮ���ȱ�Ϊˮ������ԭ���� ��B�е��ˮ�����в�������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com