̼������;�dz��㷺���ǹ�ҵ��������Ҫ����ԭ�ϣ��ҹ���ѧ�Һ�°���1921�괴���������ġ������Ƽ����������������˽����й�������Ƽ��������Ҫ�����������£�
��1��������ȡ̼����泥�NH
3+CO
2+H
2O=NH
4HCO
3���÷�Ӧ�Ļ�����Ӧ����Ϊ
��
��2���ڶ�������ʳ����NH
4HCO
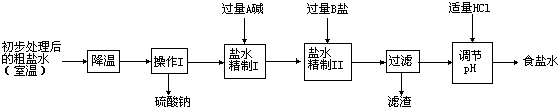
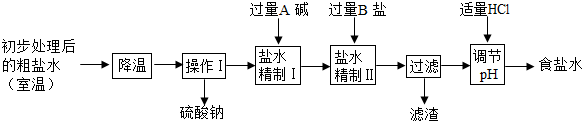
3��Ӧ��ԭ��ʳ����Դ�ڹ���IJ����壬�Ӻ�ˮ����ȡ���Σ��ô�����Ҫ�����Ȼ��ƣ��������������Ȼ��ơ��Ȼ�þ�������ƣ�Ϊ�õ�������ʳ��ˮ������ˮ�������¾������̣�

�ٴӴ���ˮ�����������ƾ�����ԭ����
��
�ڼ�A���ɼ��ص�ʯ��ʯ��Դ�õ��ģ�������ļ�A�ʹ���ˮ�е����ʷ�Ӧ�ķ���ʽΪ
��
�ۼ������B��
��������������
��
�ܲ������ϵ�֪NaCl��NH
4HCO
3��NaHCO
3��NH
4Cl��30��ʱ���ܽ�����±���ʾ��
| �¶� | NH4Cl | NH4HCO3 | NaHCO3 | NaCl |
| 30�� | 41.1g | 27.0g | 11.1g | 36.3g |
��3������������̼�����ƣ�2NaHCO
3
Na
2CO
3+CO
2��+H
2O��


 Na2CO3+CO2��+H2O��
Na2CO3+CO2��+H2O�� 

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д�



 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��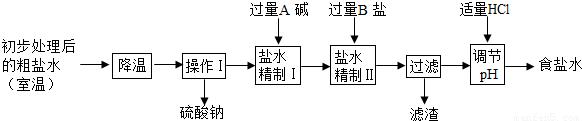

 Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��