
ij�о�С����������ᾧ����Ʒ�ֽ���ﲢ�ⶨ�������������������ʲ����뷴Ӧ�������ᾧ�壨H2C2O4?2H2O�����������ʼ�����
��1�����ȷֽ���ᾧ�������˵�װ���� ����ͼ1��ĸ��ţ���
��2��ͼ2����֤�ȷֽ�����к�CO��CO2��װ��
������a��b�����Ʒֱ��� �� ��
��֤������CO2�������� ��֤������CO�������� ��D�з�Ӧ�Ļ�ѧ����ʽ�� ��
��װ��A�������� �����ҵ������� ��
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�����������������������·�����
| �۵� | �е� | ���ȶ��� | ��Ӧ |
| 101��C��102��C | 150��C��160��C���� | 100.1��Cʧȥ�ᾧˮ��175��C�ֽ��CO2��CO��H2O | ��Ca��OH��2��Ӧ������ɫ������CaC2O4�� |
��1��c
��2�����ձ����ƾ���
��B�г����ʯ��ˮ����ǣ�D�к�ɫ�����ɺ�ɫ CO+CuO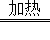 Cu+CO2
Cu+CO2
�۳�ȥ������������ֹ�Զ�����̼�ļ���������ţ��ռ�һ����̼����ֹ��Ⱦ����
��3�������ɵ�ͭ�ֱ����� ���Ϻ� 0.79g 90%
���������������1��������۵�ϵͣ����������ۻ�����cװ�ü��Ȳ���ʱ�����������������������Ȳ��ᣮ
���c��
��2��������a��b�����Ʒֱ����ձ����ƾ��ƣ�
����ձ����ƾ��ƣ�
��֤������CO2�������ǣ�B�г����ʯ��ˮ����ǣ�֤������CO�������ǣ�D�к�ɫ�����ɺ�ɫ��
���B�г����ʯ��ˮ����ǣ�D�к�ɫ�����ɺ�ɫ��
D������ͭ��һ����̼�ڼ���ʱ��Ӧ������ͭ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ�ǣ�CO+CuO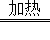 Cu+CO2��
Cu+CO2��
���CO+CuO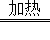 Cu+CO2��
Cu+CO2��
��װ��A�������ǣ���ȥ������������ֹ�Զ�����̼�ļ���������ţ����ҵ������ǣ��ռ�һ����̼����ֹ��Ⱦ������
�����ȥ������������ֹ�Զ�����̼�ļ���������ţ��ռ�һ����̼����ֹ��Ⱦ������
��3����һ����̼���ַ�Ӧ�����ɵ�ͭ���±����������ض��ܹ����¼������ʵ������ʵ��ֵƫ�ͣ�
������ɵ�ͭ�ֱ�������
�ڸ��������Һ����ɫ�Ϻ�ɫ�ģ�
����Ϻ죮
25.00g3.16%KMnO4��Һ��KMnO4������Ϊ��25.00g��3.16%=0.79g��
���0.79��
��10.00g��Һ�к����ᾧ�������ΪX��
��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��֪��
5H2C2O4?2H2O��5H2C2O4��2KMnO4��
630 316
X 0.79g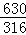 =
=
X=1.575g��
50.00g��Һ�к����ᾧ�������Ϊ��1.575g��5=7.875g��
���ᾧ�����������Ϊ��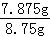 ��100%=90%��
��100%=90%��
����Ʒ�в��ᾧ�����������Ϊ90%��
���㣺ʵ��̽�����ʵ���ɳɷ��Լ���������������ļ�������ӷ�����
�����������漰��ѧ����ʽ����д��ʵ��������жϡ����ݻ�ѧ����ʽ���м���ȷ����֪ʶ���ǵ��͵��ۺ��⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�����и�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ���[Ca10(PO4)6(OH)2]��ʽ���ڣ�����Է�������Ϊ1004��ţ�̺��Ʒḻ�������գ���ţ���иƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֡�����ϸ�Ķ���ش��������⣺
��1����װ��ǩ��֬����3.3g����ָ100mLţ���к�֬������������3.3g����ôһ��ţ�̺������� g��������0.01g����
��2�����ǻ�������и�Ԫ�ص���������������Ϊ0.1%����
��3��������ÿ��������Ҫ0.6g�ƣ�����Щ����90%����ţ�̣���һ����ÿ������Ҫ�ȶ��ٺ�ţ�̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�ҹ���̨ʹ�ö�����Ϊȼ�ϵij��пͳ���Ч�ؽ���˹�����ð���̵����⣮
��1����֪�����ѵĻ�ѧʽΪC2H6O�������ѵ���Է��������� ������̼����Ԫ�ص�������Ϊ ��
��2���������ڿ�������ȫȼ�����ɶ�����̼��ˮ���������ȫȼ��92g��������Ҫ�������ٿˣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ϊ����ȣ�ݣ����������ʱ����ľ�Ǵ���C5H12O5���������ǣ�
��1��ľ�Ǵ�����Է�������Ϊ ��
��2��һ��ľ�Ǵ��������� ��ԭ�ӣ�������̼���⡢��ԭ�ӵĸ�����Ϊ ��
��3��ľ�Ǵ���̼Ԫ�ص���������Ϊ �����������0.1%����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
Ϊ�ⶨһƿʧȥ��ǩ�Ĺ���������Һ����������������ijͬѧȡ50.0g����������Һ�����ձ��У�Ȼ�����2.0g�������̣��Ȳ��ٷų����壬�Ƶ��ձ���ʣ�����ʵ�������Ϊ50.4g�����㣺
��1��������������Ԫ�غ���Ԫ�ص�������Ϊ�� ����
��2���˹���������Һ������������������д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��8.1g����п�������100gϡ�����У�ǡ����ȫ��Ӧ���Լ��㣺
�ٿ���������п���ٿˣ�
������ϡ������������������Ƕ��٣�����ѧ����ʽ��ZnO+H2SO4=ZnSO4+H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ͭþ�Ͻ���������ĵ����ԣ��������ɻ����ߵȵ�����ϣ����ⶨ�Ͻ����ɣ�����Ԫ�غ��Բ��ƣ�����������ʵ�飺ȡͭ�Ͻ�20g�����ձ�����280gϡ�����4�μ����ձ��У���ַ�Ӧ���ʣ������������¼���£�����㣺
| ���� | 1 | 2 | 3 | 4 |
| ����ϡ��������/g | 70 | 70 | 70 | 70 |
| ʣ���������/g | 18.2 | 16.4 | 14.6 | 13.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
������ѧ�Һ�°����������Ƽ��Ϊ�ҹ���ѧ��ҵ�ķ�չ�������ܳ����ף�ijʵ��С��ȡ�������Ȼ������ʵĴ�����Ʒ14.4g�����뵽ʢ��100gϡ������ձ��У�ǡ����ȫ��Ӧ����ʱ�ձ��ڸ����ʵ�������Ϊ110g���Լ��㣺
��1����Ӧ���ɶ�����̼�� ���ˣ�
��2������ϡ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���ſ������ռ�����ʱ��Ϊ�˾������ų�����ƿ�еĿ����������ܿ�Ӧ���뵽����ƿ�IJ�λ��ȷ���ǣ� ��
| A��ƿ�� | B���в� | C��ƿ�� | D������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com