
��7 �֣���ˮѡ�����ҹ��Ŵ��Ͷ���������һ���������ѡ���ӵķ�����ũҵ�����ϳ����������� 15%��20%���Ȼ�����Һ��ѡ�֡�Ϊ�˲ⶨij�Ȼ�����Һ�Ƿ����Ҫ��ȡ����Һ70g������һ���������������� AgNO3��Һ 100g��ǡ����ȫ��Ӧ�����˷��������������Ϊ28��7g��
��1���������Ȼ�����Һ�IJ��������У��ܽ�ʱ��Ҫ�õ����������������� ��
��2����Ӧ��������Һ������Ϊ g��
��3��ͨ������ȷ�����Ȼ�����Һ�Ƿ����ѡ��Ҫ����������ȷ��0��1%��
��1�������ܽ� ��2��141��3 ��3������Ҫ��
���������������1���������Ȼ�����Һ�IJ��������У��ܽ�ʱ��Ҫ�õ����������������ǣ������ܽ�
��2����Ӧ��������Һ���������ڷ�Ӧǰ�������ʵ�������ȥ���ɵ����塢����������ˮ���ʵ����������Է�Ӧ��������Һ������=70g+100g-28��7g=141��3g
��3�����ݻ�ѧ��Ӧ��AgNO3+NaCl="=" NaNO3 + AgCl���г���AgCl��NaCl��������ϵ�����NaCl����������������Ȼ�����Һ����������������15%��20%��Ƚ�
�⣺����Ȼ�����Һ���Ȼ��Ƶ�����Ϊx
AgNO3+NaCl="=" NaNO3 + AgCl��
58��5 143��5
x 28��7g
58��5��143��5=x��28��7g
x=11��7g
��Һ���Ȼ��Ƶ���������=11��7g/70g��100%=16��7%
15%��16��7%��20%������Ҫ��
�𣺸��Ȼ�����Һ����ѡ��Ҫ��
���㣺���ݻ�ѧ����ʽ����


 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�ҹ��Ƽҵ������������°�̽�������ˡ������Ƽ���������������漰����Ҫ��ѧ��Ӧ���£�
��NH2��CO2��X��NH4HCO3
��NH4HCO3��NaCl��NH4Cl��NaHCO3��
��2NaHCO3 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��ش��������⣺
��1����Ӧ����X�Ļ�ѧʽΪ ��
��2����ȥ����Na2CO3��ĩ��������NaHCO3�ķ����� ��
��3����ҵ�����к����Ȼ��ƣ�ȡ55g��ҵ��������м���269.5gϡ���ᣬǡ����ȫ��Ӧ������22g������̼����
�ٹ�ҵ������̼���Ƶ�������������������������0.1%��
�ڷ�Ӧ����Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣�Ϊ�ۺ��������������еĸ���ƷCaSO4��ij����������������Ʊ�(NH4)2SO4�Ĺ������̣�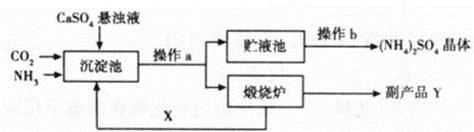
��1���������У��ڳ����ط����Ļ�ѧ��ӦΪCO2+2NH3+CaSO4+H2O==CaCO3��+ (NH4)2SO4
������¯�з����Ļ�ѧ��Ӧ����ʽΪ ���ù����п�ѭ��ʹ�õ�XΪ (�ѧʽ)������ƷY�к���; (��һ�ּ���)��
��2������Һ���л��(NH4)2SO4����Ҫ���в���b������b�� ��������һ�����������Һ���У��γ�30��ʱ(NH4)2SO4�ı�����Һ�����ʱ������������Ϊ ����֪30��ʱ����淋��ܽ��Ϊ78g����
��3�����Ʊ�6.6t (NH4)2SO4���壬��������ҪCaSO4���ٶ֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
С�������ۺ�ͭ����ɵ�7g��������ʢ��121.6gϡ������ձ��У��պ���ȫ��Ӧ���ձ������ʵ�������Ϊ128.4g�����ܼ��㣺
��1��ԭ����������۵�������
��2����Ӧ��������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��9�֣�ʵ������һƿ������Һ����ʦ��С��ͬѧ��Ʒ����ⶨ�÷�Һ�����������������С��ͬѧ��ȡһ�ྻС�ձ�����������Ϊ18.2g��Ȼ�������е������������Һ�������������Ϊ33.2g��֮��һö����Ϊ10.8g������������ɰֽ��ĥȥ�����⣩�����С�ձ��з�Ӧ�����������治�������ݲ������ٴγ�����������Ϊ43.9 g��
��ش��������⣺
��1����Ӧ�в���������������� ��
��2������÷�Һ�����������������д��������̣�����������һλС��������6�֣�
��3���������������δ�������Լ�������Ӱ���� (ѡ�ƫ����ƫС��������Ӱ�족)��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(3��)����β��ϵͳ��ʹ�ô�ת�������ɽ���CO��NO���ж�������ŷţ��䷴Ӧ��ѧ����ʽΪ��2CO+2NO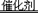 2CO2+N2������2.8gCO��ת��ʱ������ͬʱ��ת����NO��������
2CO2+N2������2.8gCO��ת��ʱ������ͬʱ��ת����NO��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(4��)ijУ��ѧ��ȤС���ͬѧΪ����ȡ������þ������ȡ9.5g�Ȼ�þ����ȫ���ܽ���40.5gˮ���Ƴɲ�������Һ��Ȼ�������м���55.8gij������������������������Һǡ����ȫ��Ӧ������㣺
(1)�Ƶõ�������þ��������
(2)��Ӧ��������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ʵ����������غͶ���������ȡ����������һ��ʱ���ʣ���������10g��������������ȫ��Ӧ��(�����������ٱ仯ʱ)�����Ϊ9.04g���ٽ������ˮ����ܽ⡢���ˡ�����õ�1.59g��ɫ���塣��10gʣ�������������Ԫ�ص���������Ϊ
| A��7.1�� | B��35.5�� | C��28.4�� | D��42.6�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�ҹ������������ʪ����ͭ���䷴Ӧ�Ļ�ѧ����ʽΪ��Fe+CuSO4�TFeSO4+Cu��������32kg��ͭ��Ҫ������Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com