
����Ŀ����Һ������������������ʮ����Ҫ�����á���ش��������⣺
��1��������Ϊ���ʵ���___________��
A ֻ�й��� B ֻ��Һ��
C ֻ������ D ���塢Һ�塢���嶼����
��2������100g��������Ϊ16%���Ȼ�����Һ�������Ȼ��Ƶ�����Ϊ___________��ˮ�����Ϊ_________mL ��ˮ���ܶȽ��ƿ���lg/cm3����
��3���ס������ֲ����ᾧˮ�Ĺ������ʵ��ܽ����������ͼ.t1��ʱ�������ʵ��ܽ����___________��t2��ʱ�����Ӽ����������ʵı�����Һ�������������Ĺ��壬���������϶�ˮ����___________ �����������������������ʵı�����Һ��
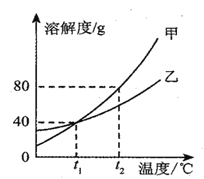
��4������˵����ȷ����___________��
A.�������ʵı�����Һ��Ϊ��������Һ�������ʵ���������һ����С
B.��t2��ʱ�ס������ʵ���Һ���µ�t1��������Һ������һ�����
C.����һ������������������������Һ������ȡˮʱ���Ӷ�����������������ȷ�������Ƶ�����Һ�����ʵ�����������ƫ��
ѡ�� | x | y |
A | ˮ | ������ |
B | ˮ | ����� |
C | Ũ���� | ˮ |
D | ϡ���� | þ |
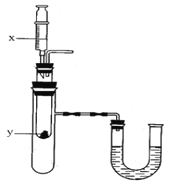
��5������ͼ��ʾװ�ã���Һ��Xע��װ�й���y���Թ��У��ᵼ��U�ι����Ҷ�Һ�����ߡ�����ܵ������______________________��
���𰸡�D 16g 84mL 40g �� C AD
��������
��1�����塢Һ�塢���嶼������Ϊ���ʣ��γ���Һ������D��
��2������100g��������Ϊ16%���Ȼ�����Һ��������������=��Һ����������������������֪�����Ȼ��Ƶ�����=100g��16%=16g����Ҫˮ������=100g-16g=84g���ٸ������=![]() ��֪��Ҫˮ�����=
��֪��Ҫˮ�����=![]() =84cm3=84mL������84mL ��
=84cm3=84mL������84mL ��
��3�������ܽ�����߿�֪��t1��ʱ�������ʵ��ܽ����40g������40g��t2��ʱ�������ʵ��ܽ�ȴ��������ʵ��ܽ�ȣ��������Ӽ����������ʵı�����Һ�������������Ĺ��壬���������϶�ˮ���������ʵı�����Һ�������ң�
��4��
A�������ʵ��ܽ�����¶����߶����������ʵı�����Һ��Ϊ��������Һ���ɲ��������ܼ��������¶ȵķ����������ܼ����������ʵ���������һ����С�������¶ȵķ��������������������䣬�ʲ��������⣻
B��û��ָ����Һ�������Լ���Һ�Ƿͣ���t2��ʱ�ס������ʵ���Һ���µ�t1�棬����ȷ������Һ��������ϵ���ʲ��������⣻
C����ȡˮʱ���Ӷ����ᵼ��ʵ����ȡˮ�����ƫС��������������ȷ�������Ƶ���Һ�����ʵ�����������ƫ�ߣ��ʷ������⣻����C��
��5��
��Һ��xע��װ�й���y���Թ��У��ᵼ��U�ι����Ҷ�Һ�����ߣ�����Ҫʹװ����ѹǿ����ʹx��y��Ӧ�������壬��Ӧ���Ȼ��ܽ����ʹ�¶����ߣ�
A�������ƺ�ˮ��Ӧ�����������ƣ��ų��������ȣ�ʹװ����ѹǿ���ʷ������⣻
B�����������ˮ��Һ�¶����Խ��ͣ�װ����ѹǿ��С���ʲ��������⣻
C��Ũ��������ˮ�ų���������Һ�¶����ߣ�װ����ѹǿ����������y�ǹ��壬�ʲ��������⣻
D��ϡ�����þ��Ӧ����������ͬʱ�ų�������װ����ѹǿ���ʷ������⣻����AD��


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʢ�е�����������������ϡ�������ֻ�ձ�,����������ƽ����������,��ƽƽ��,�������ձ��м���10gCaCO3��ǡ����ȫ��Ӧ����Ҫʹ��ƽ����ƽ�⣬�������ձ���Ӧ���������������
A. 5.6g����пB. 10gþ������þ
C. 10g̼��þD. 10g̼���ƺ�̼��п
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2019��Ϊ��Ԫ�����ڱ�������Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߡ�
��1��ԭ������Ϊ1~18��Ԫ����Ԫ�����ڱ��е�λ�����±���ʾ��
��1���� | H | He | ||||||
��2���� | Li | Be | B | C | N | O | F | Ne |
��3���� | Na | Mg | Al | Si | P | S | Cl | Ar |
��Al����_____�����������������ǽ�������Ԫ�أ���ԭ�ӵĺ˵����Ϊ______
��Naԭ�ӵĽṹʾ��ͼΪ ���������ӵĺ��������Ϊ_______��
���������ӵĺ��������Ϊ_______��
�����ڱ�����Ԫ�ص��й���Ϣ��ͼ��ʾ��ͼ����30.97������ʾ�ĺ�����_______.

��2���������ڱ����ֵĽṹ������֮��Ĺ�ϵ��ijͬѧ������ͭ���仯����������ϼ�һ�����������ϵͼ��
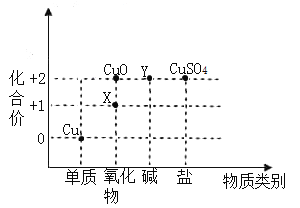
��д������X�Ļ�ѧʽ��_____��
��д��������Yת��Ϊ![]() �Ļ�ѧ��Ӧ����ʽ��______.
�Ļ�ѧ��Ӧ����ʽ��______.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ч��ת�������ɽ�����β���е��ж����崦��Ϊ����Ⱦ�����壬ͼΪ�÷�Ӧ����ʾ��ͼ������˵����ȷ���ǣ� ��
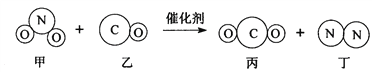
A. ���ɵı��Ͷ��ķ��Ӹ�����Ϊ1��1
B. ��Ӧǰ��̼�͵��Ļ��ϼ۷����˸ı�
C. ��Ӧǰ��Ԫ�ص�����û�иı䣬ԭ�ӵĸ��������˱仯
D. �Һͱ���Ԫ�������ͬ���������ǵĻ�ѧ������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̽��ʵ�鲻�ܴﵽĿ����
A. 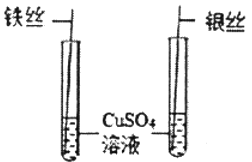 ̽��Fe��Cu��Ag�Ľ������
̽��Fe��Cu��Ag�Ľ������
B. 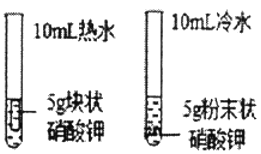 ̽��Ӱ�������ܽ����ʵ�����
̽��Ӱ�������ܽ����ʵ�����
C. 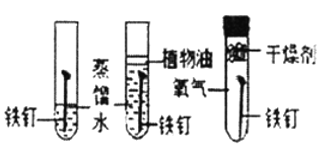 ̽�������������
̽�������������
D. 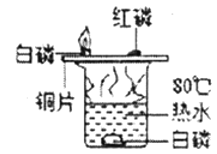 ̽����ȼ��ȼ�յ�����
̽����ȼ��ȼ�յ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±��Ǽ��ֿ�ȼ���ڳ�ѹʱ�ķе㣻
��ȼ�� | CH4 | C2H6 | C3H8 | X | C5H12 |
�е�/�� | 164 | 88.6 | 42.1 | 0.5 | 36.1 |
��1����C3H8�У�̼Ԫ������Ԫ�ص�������Ϊ_____________����������ȣ���
��2����C3H8�У�̼Ԫ�ص���������Ϊ_____________����������ȷ��0.1%����
��3��45gC2H6��_____________gC5H12����̼Ԫ��������ȡ�
��4�������ϱ��п�ȼ����ӽṹ�ϵĹ��ɣ��Ʋ�X�Ļ�ѧʽΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ŀǰ������ʹ�����Ľ�����
��1����ʶ�����Ӻ�ۿ�������_____��ɣ����ۿ�������_____���ɡ�
��2�������У�������ѹ��Ƭ�����˿������������_____�ԡ�Ϊ��ֹ����Ʒ��ʴ���ɲ��õķ�����_____.(һ�֣���
��3��ʵ���ң�ϸ��˿�������г��ȼ�յĻ�ѧ����ʽ��_____��
��4����ԣ�����X��Y��Z���ֽ�������֪��X+YSO4=XSO4+Y��Y+H2SO4������Ӧ��Y+2ZNO3=Y(NO3)2+2Z,�����з���Ҫ���X��Y��Z�ֱ���_____(����ţ���
A Zn��Mg��Ag B Mg��Fe��Ag C Zn��Cu��Ag D Fe��Zn��Ag
��5����ҵ�ϣ���1000t��������80%�ij�����ʯ�������Ͽ�������������6%������������Ϊ_____t(�����ȷ��0.1)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ʒ��������һ�ѧ����ʽ��д��ȷ����
A. ֤���������Mg![]() Ag��Mg
Ag��Mg![]() 2AgCl2MgCl2
2AgCl2MgCl2![]() Ag
Ag
B. �����ᱵ�����������ƺ�����أ�K2SO4![]() Ba(NO3)2BaSO4��
Ba(NO3)2BaSO4��![]() KNO3
KNO3
C. ��ϡ�����ȥ̿���е���������ͭ��CuO![]() H2SO4CuSO4
H2SO4CuSO4![]() H2O
H2O
D. ��������������θ�����֢��Ca(OH)2![]() 2HClCaCl2
2HClCaCl2![]() 2H2O
2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ������֮Դ������֮���������౦�����Ȼ��Դ������ÿ���˶�Ҫ����ˮ������ˮ����Լ��ˮ��
��1�������п��Բ���______����Ӳˮ����ˮ������ͨ��______�ķ�������ˮ��Ӳ�ȡ�
��2���������ɹ��������õĵ�ˮ��������1%������ˮ��Դ��ÿ����������κ����������������ڽ�Լ��ˮ����______
A ϴ�������ʱ��������ͷ B ����Ϸ�ˮˢ��
C ������ˮ����Ϊ���ϳ�ˮ���� D ũҵ���ֽ����õιࡢ���
��3������ˮ����ˮ���ˮ����������ˮ��������������ҪΪ��ˮ���ˮ��������______��������______������ˮ��ѡ����ţ�
A ���� B ���� C ���� D ����
��4������ˮ���������г��ᷢ�����»�ѧ��Ӧ���䷴Ӧ���۹��̿�����ͼһ������ʾ
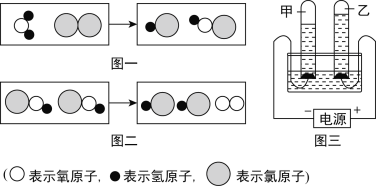
������ʾ��ԭ�ӣ�����ʾ��ԭ�ӣ�![]() ��ʾ��ԭ�ӣ�
��ʾ��ԭ�ӣ�
������ͼ�������ֵ������У�����______�ֺ�����Ԫ�صĻ����
�ڴ�ͼ�����ǿ�֪���ڻ�ѧ��Ӧ�з�Ӧǰ��______�������䣻
��ͼ���еĻ�ѧ��Ӧ���ڻ�����Ӧ�����е�______��
��5������ͼ����ʾ��װ�ý��е��ˮʵ�飬������������ȷ����______��д���û�ѧ��Ӧ�ı���ʽ______��
����ˮ�м����������������ƣ���ʹˮ������������ٶȱ�죻
�ڼס������Թ����ռ���������������ԼΪ2:1��
�ۼ��Թ��ڲ�����������ȼ�գ�
�����Թ��ڵ�������п�ȼ�ԣ�
����ʵ���֪ˮ��������Ӻ������ӹ��ɵġ�
��6����ͼΪ����ʵ�������
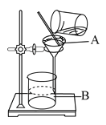
������A������______��
����ָ��ͼ�еIJ�������______��
����ͼB��ʾ��˵������Ҫ��______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com