
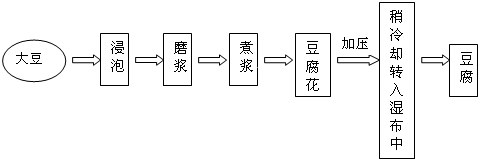
| �ɷ� | ˮ | ������ | ֬�� | ���� | �� | �� | �� | ά����B1 | ά����B2 |
| ��������% | 90.0 | 4.7 | 1.3 | 2.8 | 0.24 | 0.064 | 1.4 | 0.00006 | 0.00003 |


 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
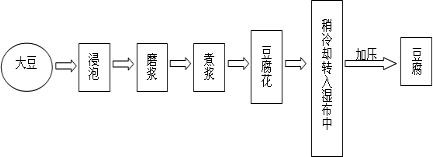
| �ɷ� | ˮ | ������ | ֬�� | ���� | �� | �� | �� | ά����B1 | ά����B2 |
| ��������% | 89.3 | 4.7 | 1.3 | 2.8 | 0.24 | 0.064 | 1.4 | 0.00006 | 0.00003 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �ɷ� | ˮ | ������ | ֬�� | ���� | �� | �� | �� | ά����B1 | ά����B2 |
| ƽ����������/% | 89.3 | 4.7 | 1.3 | 2.8 | 0.24 | 0.064 | 1.4 | 0.00006 | 0.00003 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
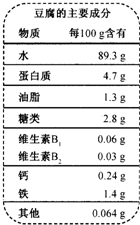 ��2008?��������ģ���ƶ������й��Ŵ���һ����Ҫ�����������ȫ���ѳ�Ϊ���ܻ�ӭ��ʳƷ������Ҫ�ɷ���ͼ��ʾ��
��2008?��������ģ���ƶ������й��Ŵ���һ����Ҫ�����������ȫ���ѳ�Ϊ���ܻ�ӭ��ʳƷ������Ҫ�ɷ���ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ˮ��������Ҫ�ɷ� ���� ÿ100g���� |
| ˮ 89.3g |
| ������ 4.7g |
| ��֬ 1.3g |
| ���� 2.8g |
| ����B1 0.06mg |
| ����B2 0.03mg |
| �� 0.24g |
| �� 1.4g |
| ���� 0.064g |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com