
��ѡ�á�>����<��������ա�
��1������������ɷֵ�������������� ϡ�����壻
��2��ͨ��״���µ�Һ�������100mL�ƾ���100mLˮ��� 200mL��
��3�������£�1g����ر�����Һ�м���1g����ع����ܵõ���Һ������ 2g
��4����ͬ������ϸ��˿��������ʣ�������ˮ��������˿ ��ʳ��ˮ��������˿
��5��1g�����1g��������ȫȼ�պ����ɶ������������ 2g
��6���۵㣺��ºϽ� ����ɽ���
> ��< ��<��<��=��< ��
�������������
��1������������ɷֵ��������������ռ21%��ϡ������0.94% ��
��2�����Ӽ��м������100mL�ƾ���100mLˮ������С��200ml��
��3��������Һ�Dz��ܼ����ܽ�����ʵ���Һ����1g����ر�����Һ�м�������ع��岻�ܼ����ܽ⣻
��4��ʳ��ˮ�ܼ����������⣻
��5������������Ӧ��������Ϊ1:1����1g�����1g��������ȫȼ�պ����ɶ������������Ϊ2g��
��6���Ͻ���۵��������Ĵ��������۵�ͣ�
���㣺���Ĺ�ϵ


 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��9�֣�ˮ����Һ��������������Ҫ�����ã�
��1������ˮ�����õľ�ˮ�����г�������_________�������������ȣ����ճ������г���������ˮ��Ӳ�ȵķ�������_________����ˮ��һ�ֱ������Ȼ��Դ�����DZ��밮��ˮ��Դ��Ԥ��ˮ��Ⱦ�Ĵ�ʩ����_________����дһ�����ɣ���
��2����ͼ�Ǽס����������ʵ��ܽ�����ߣ��ش��������⣺
����_________��ʱ���ס����������ʵ��ܽ����ȣ�
����������к���������ʱ������_________�������ᴿ�ף�������ᾧ������ȴ�ᾧ������
��t2���õ������ļס��������������üס��ҵı�����Һ��Ҫˮ����������_________���ң��������������=��������2�֣�
������һ���������������ļ�������Һ�����²����лᵼ����������Һ������������ƫ�͵�����_________������2�֣�
A����Ͳ��ȡˮʱ���Ӷ���
B����ϵ���Һ��ϸ��ƿת��ʱ����
C��������к������ʣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�ǵ��ˮ��ʵ��װ��ͼ������������˽��֪ʶ��գ�
��1��AΪ ����
��2����������ļ��鷽�� ��
��3�����1molˮ����O2������Ϊ���ٿˣ�(д���������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���淶��ʵ�������ʵ��ɹ���ǰ�ᣮ��ش𣺢�����������ʱ���Ѳ������ܽ����ز��뵥����Ƥ���Ĵ�ʩ��___________������ȡ7mLϡ���ᣬӦѡ��___________ mL����Ͳ���۽�ͷ�ι��ù���Ӧ___________����ȥ��ȡ����ҩƷ����2��ͼ��ʵ������������ȡ���ռ������װ��ͼ��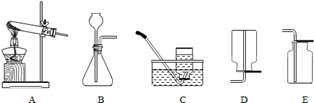
ʵ�����ø����������ȡ������Ҫ��õ��ϴ�����������Ӧѡ��ķ������ռ�װ��Ϊ___________������ĸ�����ڷ���װ�õ��Թܿڷ�һ������������___________���������������ȡ��������Ӧ�Ļ�ѧ����ʽΪ___________�����������ڷ�Ӧ�е�������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ������֮Դ��������������ҡ�������Һ�����ڡ��й����������������з������ҹ�ȫ����ˮˮ����Ⱦ�Ӿ硣��������ά�ֺ���Ŀɳ�����չ��ÿ�����������ʥְ����ش�������ں���ļ������⣺
(1)�����Ǿ����Դ���⣬����ˮ�еĻ�ѧ��Դ�ͺ�������Դ�⣬���̲��ŷḻ�ĺ��� ��Դ�ͺ��� ��Դ��
(2)2012��5��18�գ���ɳ�庣�����ϴ�����ೱ���ೱƵ������Ҫԭ��֮һ��ˮ�帻Ӫ�����������賤��ij���������Ҫ�ɷֵĻ�ѧʽΪC106H263O110N16P������̼����Ԫ�ص�������Ϊ ��
(3)��ˮ�����ǹ������о����ȵ����⣬�ҹ���ѧ�������ø߷���Ĥ���к�ˮ�������о���ȡ����һЩ�ɼ�����ͼ��ʾ���Ҳ�Ϊ��ˮ�����Ϊ����һ��ʱ������Ҳຣˮ���߷���Ĥ���õ��ĵ�ˮ���ɴ˷����߷���Ĥ������е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ��������һƿ���壨������Ϊ����������ڱ�ǩ�Ѿ���ʴ�������ϣ��������Ʋ������������������һ����̼�������е�һ�֡�ijͬѧ����������̽����
��ȡһ�ྻͭ˿���ھƾ��ƻ����ϼ��ȣ�ͭ˿�����ɺ�ɫ��
�ڳ��Ȱѱ�����ɫ��ͭ˿���뵽��������ļ���ƿ�в��ܱռ���ƿ���ɹ۲쵽ͭ˿����ĺ�ɫ�ֱ��������ɫ��
��1����������ѧ�Ļ�ѧ֪ʶ�����ƶϳ�������������� ��
��2����ͬѧ��Ϊ���Ҫȷ�����������������ƶϵ����壬����Ҫ��������һ��̽�������������� ���ɹ۲쵽�������� ����Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ�����������ߣ����ǵ������벻����ѧ���ʡ��������¼������ʣ���Һ�� ��ϡ������ ��ˮ �ܸɱ�����ѡ���ʵ��������������գ�
��1�������������ȼ������ ��
��2���������˹�������� ��
��3�������������ݵ��� ��
��4������Ϊ����֮Դ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ������������Դ��
��1����ȥˮ�����������ʵIJ����� �����в������������� ��
��2��ˮ����������ʷ�����ѧ��Ӧ���Ծ�һ����д����ѧ����ʽ�� ��
��3����ͼ�м��ǵ�����ˮ��ģ��װ�ã����ǵ��ˮ��װ�á�
����ˮ��״̬�仯Ϊ��Һ̬ ��̬
��̬ Һ̬�����������ˮ���ӵ� �����˸ı䡣����������ˮ����ȴ����������
Һ̬�����������ˮ���ӵ� �����˸ı䡣����������ˮ����ȴ����������
a��ˮ���ӵĵ��� b�������ݴ� c����ɢ��
�������Թܡ�2��������ļ��鷽���� ��ˮ�ֽ�����е���С���� ������ĸ���� �ɱ�ʾһ��ˮ���ӣ�
�ɱ�ʾһ��ˮ���ӣ�
���϶����š�̽��ʹ�õ�Һ�⼴Ϊ���ˮ���á���ȡ3molH2����ˮ�����ʵ���Ϊ �����ݷ���ʽ��ʽ���㣩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ������������أ�
��1����ů��������ʦ��֯ͬѧ�ǵ�����Ұ������У�ͬѧ��Я������������������������������ʳ���͡�ʳ�Ρ�ζ����ʳ�ס�ȥ�۷ۣ���Ч�ɷ�Ϊ̼���ƣ�����Ʒ��
�ٴ�ͬѧ��������ʳ������ѡ����Ϊ�����ṩ�϶�ά���ص�һ��ʳ������ ������д��ѡʳ������ƣ����ڴ������ʳ���л�ȱ�ٵ�һ�������������Ӫ�������� ������Ӫ�������ƣ�
��Я�������У�������װʳ�Ρ�ȥ�۷۵�ƿ�ӻ����ˣ��е�ͬѧ���飬��������Ʒ�е��� ��������Я������Ʒ���ƣ��Ϳ��Խ��������ֿ�����
��2�������ǣ�C6H12O6���������ɹ�������Ļ��ά����������Ҫ���������䷴Ӧ�ɱ�ʾΪ��C6H12O6+��
�� O2 6CO2+6H2O+������
6CO2+6H2O+������
�����ڷ��������ϡ����֡�����ɸ÷�Ӧ��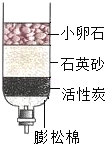
�������й�˵���У�����ȷ������ ��������ĸ��ţ���
| A���������к���24��ԭ�� |
| B��������ʳ���������������У���Ҫ�������� |
| C��ҽ���Ͽ���һ��������������������Һ��������Һ�Բ������� |
| D�����������������Ķ�����̼���ܼ�ʱ�ų����壬��ѪҺpH������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com