
����Ŀ����һ���ܱ���������������������̼��ˮ������һ��δ֪����M����һ�������³�ַ�Ӧ����÷�Ӧǰ������ʵ��������±���
���� | ���� | ������̼ | ˮ���� | M |
��Ӧǰ����/g | 100 | 1 | 1 | 46 |
��Ӧ������/g | 4 | 89 | 55 | x |
(1)���������غ㶨�ɣ�����Ϊx��ֵӦΪ_____________��
(2)δ֪����Mһ�����е�Ԫ��Ϊ______________________��
(3)��֪δ֪����M����Է�������Ϊ46���Ƴ��仯ѧʽΪ_____________��
(4)�÷�Ӧ�Ļ�ѧ����ʽΪ______________________________��
���𰸡�0 C��H��O C2H5OH C2H5OH+3O2![]() 2CO2+3H2O
2CO2+3H2O
��������
���������غ㶨�ɣ��ɱ������ݿ�֪��������̼������������89g-1g=88����ȷ��������̼�������ˮ����������������55g-1g=54g����ȷ��ˮ���������������������������100g-4g=96g������ȷ�������Ƿ�Ӧ�ͬʱ����ȷ��M�Ƿ�Ӧ����(1)M���ٵ�����Ϊ88g+54g-96g=46g�����x��ֵΪ46-46=0��
(2)��Ӧ���е�������ֻ����Ԫ�أ�����������е�̼Ԫ�غ���Ԫ��һ������M����M��һ������̼��������Ԫ�أ�����������Ԫ�ص�����Ϊ88g��![]() ��100%+54g��
��100%+54g��![]() ��100%=112g��96g��ȷ���������е�һ������Ԫ������M����M��һ��������Ԫ����
��100%=112g��96g��ȷ���������е�һ������Ԫ������M����M��һ��������Ԫ����
(3)M��̼���⡢������Ԫ�ص�ԭ�Ӹ�����Ϊ����88g��![]() ��12������54g��
��12������54g��![]() ��1������16g��16��=2��6��1������ΪM����Է�������Ϊ46������M�Ļ�ѧʽ��C2H5OH��
��1������16g��16��=2��6��1������ΪM����Է�������Ϊ46������M�Ļ�ѧʽ��C2H5OH��
(4)�÷�Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2![]() 2CO2+3H2O��
2CO2+3H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���Ҽ�������غͶ��������Ļ����28g��ȡ��������ȫ��Ӧ��ʣ���������Ϊ18.4g������㣺
��1������������������
��2��ԭ�����������ص�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ĸ��ʾ���������ʾ���H��C��O��Fe�еļ���Ԫ����ɡ�
(1)A��B����ͬԪ�������A�������к�������������д��B�ֽ�ΪA�Ļ�ѧ����ʽ��_______________��
(2)D��E�ڳ����¾�Ϊ��̬������������D������ȼ����д��Dת��ΪE�Ļ�ѧ����ʽ��_______________��
(3)M��N����ͬԪ�������M�Ļ�ѧʽΪFeaOb(a��b��Ϊ����)������Է�������Ϊ160����a��bΪ____����һ�������¿��Է������з�Ӧ��N+4D=3Fe+4E����N�Ļ�ѧʽΪ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��I��ʾ���л�ѧ���������ʣ�����C�Ǵ���ʯ����Ҫ�ɷ�,D�׳�Ϊ�ռG��һ�ֳ���������ֻ������Ԫ�ء������ʼ��ת����ϵ����ͼ��ʾ����Ӧ��������ȥ����ش��������⣺
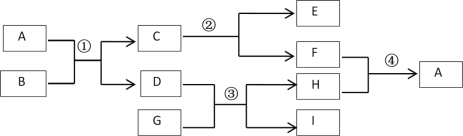
��1��B�Ļ�ѧʽΪ______________�� ��2��F��һ����;��______________��
��3������ת����û���漰�Ļ�����Ӧ������_____________��
��4����Ӧ�۵Ļ�ѧ����ʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʵ���ҳ�������ȡ�����װ�ã�
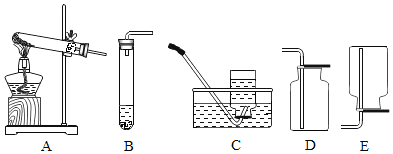
��1��ʵ���Ҳ���װ��A��ȡ����ʱ�����Թ��пɷŵ�ҩƷ��______����дһ�֣��Թܿڻ�Ҫ��һ������Ŀ����______���÷�Ӧ�����ֱ���ʽ��______��
��2���ռ���������ѡ��______��______������ĸ��װ�ã�ԭ����______��______
��3������Cװ���ռ�������������ƿ���ˮ�����Ժ���______����С�ĵذѼ���ƿ�Ƴ�ˮ�ۣ������������ϣ�
��4��ʵ����ϣ���ֹͣ�����ٽ������Ƴ�ˮ�棬��Ԥ�����ֲ���������ʲô�����______
��5������Dװ���ռ�һƿ��������μ����ռ���û�У�______
��6���ù���������Һ�����������ȡ������ѡ��______������ĸ��װ�ã�
��7��������������ˮ�����ܶȱȿ���С��ʵ���ҳ��ü��ȹ�������狀�����ʯ�ҵĻ��������ȡ������Ӧ��______������ĸ������װ�ã��ռ����������______������ĸ���ռ�װ�ã�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���� 20��ʱ�����������ļס������ֹ���ֱ����ʢ�� 100 g ˮ���ձ��У���ֽ�������������ͼ��ʾ���ס��������ʵ��ܽ����������ͼ��ʾ��������˵����ȷ���ǣ� ��
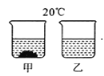
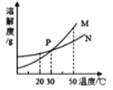
A. �ձ����е���Һ��N ��Һ
B. �ձ��ҵ���Һһ���Dz�������Һ
C. �ձ�����Һ���µ� 30��ʱ,�ձ��ײ����в������ʲ��ܽ�
D. ���ձ����ձ��ҵ���Һ�����µ� 30��ʱ���ʵ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�����������ʼף���Է��������� 90������������ȫȼ�գ������� 6.4 g ������ͬʱ������ 5.6 g CO��4.4 g CO2 �� 5.4 g H2O�������жԼ�˵������ȷ���ǣ� ��
A. ֻ����̼��������Ԫ��
B. һ������̼����Ԫ�ء����ܺ�����Ԫ��
C. ̼Ԫ�ص���������Ϊ 40%
D. ����ͬ�����ļ��� 8.6 g ��������ȫȼ�գ�ֻ���ɶ�����̼��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E�ֱ��dz��л�ѧ���������ֲ�ͬ�������ʣ�����A�ڹ�ҵ�������Ʋ�����B�Ĺ����E��Ũ��Һ������ʵ���ҵĸ������F��һ�ֺ�ɫ���壬����֮�������ͼ��ʾ�Ĺ�ϵ(ͼ����������ʾ���˵������ܷ�Ӧ����������ʾ���ʼ����ת����ϵ���������ʺͷ�Ӧ����δ���)���밴Ҫ��ش��������⣺
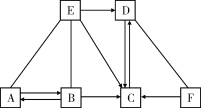
(1)д������D��F�Ļ�ѧʽ��________��__________��
(2)д��A��E�Ļ�ѧ����ʽ��__________________________________��
(3)��B��Һ��E��Һ��ֻ�ϣ�������ҺpH��7����ʱ��Һ�д������ڵ���������_________(�û�ѧ���ű�ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ������֮Դ����������й�ˮ�����ۡ�
��1�����û���̿��________�Գ�ȥ��ˮ�еij�ζ��
��2����ʵ�����п���________�ķ�����ȥ��ˮ�е���ɳ�Ȳ����Թ������ʡ������ճ������________�����������Ϸ����õ��ġ�ˮ���Dz���Ӳˮ��
��3�����෴Ӧ����ˮ�μӻ����ɡ���дһ����ˮ���ɵķֽⷴӦ��ѧ����ʽ��________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com