
| 106 |
| x |
| 74 |
| y |
| 100 |
| 100g |
| 80 |
| z |


 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?������һģ��NaCl ��KNO3�ڲ�ͬ�¶�ʱ���ܽ�����£��ش��������⣮
��2012?������һģ��NaCl ��KNO3�ڲ�ͬ�¶�ʱ���ܽ�����£��ش��������⣮ | �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| �ܽ��/g | NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
| KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
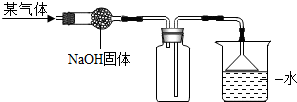 ��2012?������һģ����ͼ���ռ�ij�����װ�ã���ʵ��װ���Ʋ��������й����ʿ�����ȷ���ǣ������� ��2012?������һģ����ͼ���ռ�ij�����װ�ã���ʵ��װ���Ʋ��������й����ʿ�����ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com