A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO
2�ķ����ͺ�H
2SO
4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ���
��1�����������������ԭ��
��
��2����һ��˵������Ի�����ɵ�Σ����
��
��3�����������Ǵ�����Ⱦ�����ˮ������Һ��pH
7������ڡ�����С�ڡ����ڡ������������������������������Һ�����գ���д���÷�Ӧ�Ļ�ѧ����ʽ��
��
��4������ij��ѧ����С�������������������д�ʩ������Ϊ���в�����
A�������᳧�������
B�����黷��������������
C�������᳧�ų��ķ����е�SO
2�������ŷ�
D�������;�����ý�����ȼ��
��5��������ʯ�����������᳧�ų������Է�ˮ������ԭ���Ļ�ѧ����ʽ��
��
��6��Ũ����Ū�����Ϻ�Ӧ������ˮ��ϴ��Ȼ��Ϳ��̼�����ƣ�����ϡ����Ū�����ϣ�
�����Ҫ������Ҫ������������������
��
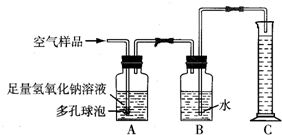
B���ݱ�������2005��1��1���𣬹���ʡ���ػ������Ž���ʵ��������Ⱦʵʩ�ϸ�Ļ�����ܣ�����ѧУ��ѧʵ����Ҫ�ŷųɷָ��ӵ���Ⱦ�����Ҳ����Ϊ������ܷ�Χ��ijУ��ѧ��ȤС���ͬѧ�ڼ�ʵ�����н����������ֱ���̼��������Ӧ��ʵ���Ϊ�˽��ʵ�����������Կ����ɷ���ɵ�Ӱ�죬�������������ʵ��װ�ý���ʵ�飨ͼ�ж�����ݵ������ǣ�������������Һ�ĽӴ������ʹ��Ӧ��ֽ��У�����ش��������⣺
��1��ʵ����û������Ⱦ����ʵ�����н��У�ȡ��������ʵ�����п�����Ʒ�ķ�����
��
��2����ȡ�õĿ�����Ʒ����ͼ��ʾ��װ�ý���ʵ��ⶨ��װ��A��������
�����з�����Ӧ�Ļ�ѧ����ʽΪ
��װ��B�е�������
��װ��C��������
��
��3����ͨ�������Ʒ100mL��ʵ���������Ͳ��Һ������Ϊ99mL��������Һ����Բ��ƣ���˵��װ��
��������1mL���壮����Щ��������ŷŵ������У������
����Ի�����ɲ���Ӱ�죻
��4����д����������ѧʵ��ʱ������ʵ��Ի�����Ⱦ��һ��������
��

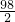 =
= ��
��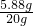 ��100%=29.4%��
��100%=29.4%��

 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
 ijУ��ѧ����С���ͬѧ��ҪһЩ��������ѧʵ�飮����һƿδ������Ũ���ᣬ��ǩ��ͼ�����Ķ���ǩ�ϵ�˵�����ش��������⣮
ijУ��ѧ����С���ͬѧ��ҪһЩ��������ѧʵ�飮����һƿδ������Ũ���ᣬ��ǩ��ͼ�����Ķ���ǩ�ϵ�˵�����ش��������⣮
 A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ���
A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ��� ijУ��ѧ����С���ͬѧ��ҪһЩ��������ѧʵ�飮����һƿδ������Ũ���ᣬ��ǩ��ͼ�����Ķ���ǩ�ϵ�˵�����ش��������⣮
ijУ��ѧ����С���ͬѧ��ҪһЩ��������ѧʵ�飮����һƿδ������Ũ���ᣬ��ǩ��ͼ�����Ķ���ǩ�ϵ�˵�����ش��������⣮