
| ʱ��/h | 1 | 1.5 | 4 | 8 | 12 | 24 | 48 | 60 | |
| ��ˮ����/g | Ũ���� | 1.6 | 2.2 | 6.2 | 10.3 | 14.0 | 20.9 | 29.2 | 32.1 |
| ϡ���� | 1.2 | 1.5 | 3.5 | 5.9 | 8.1 | 12.9 | 19.5 | 21.0 | |
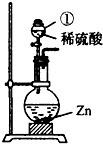
| v��1.84g/cm3��98% |
| v��1.84g/cm3+v��1g/cm3 |
| v��1.84g/cm3��98% |
| v��1.84g/cm3+v��1g/cm3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ʱ��/h | 1 | 1.5 | 4 | 8 | 12 | 24 | 48 | 60 | |
| ��ˮ����/g | ����� | 1.6 | 2.2 | 5.2 | 10.3 | 14.0 | 20.9 | 29.2 | 32.1 |
| ������ | 1.2 | 1.5 | 3.5 | 5.9 | 8.1 | 12.9 | 19.5 | 21.0 | |
| ��Һ�п��ܺ��е����� | ���ʵ����֤��ʵ�鲽�衢����Ӧ�Ľ��ۣ� |
SO42- SO42- |
ȡ������Һ���������ᱵ��Һ�����а�ɫ����������˵����Һ�к���SO42-�����ް�ɫ����������˵����Һ�в�����SO42-�� ȡ������Һ���������ᱵ��Һ�����а�ɫ����������˵����Һ�к���SO42-�����ް�ɫ����������˵����Һ�в�����SO42-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?���ݣ�ijѧϰС���������������о���
��2011?���ݣ�ijѧϰС���������������о���| ʱ��/h | 1 | 1.5 | 4 | 8 | 12 | 24 | 48 | 60 | |
| ��ˮ����/g | Ũ���� | 1.6 | 2.2 | 6.2 | 10.3 | 14.0 | 20.9 | 29.2 | 32.1 |
| ϡ���� | 1.2 | 1.5 | 3.5 | 5.9 | 8.1 | 12.9 | 19.5 | 21.0 | |
| v��1.84g/cm3��98% |
| v��1.84g/cm3+v��1g/cm3 |
| v��1.84g/cm3��98% |
| v��1.84g/cm3+v��1g/cm3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijѧϰС���������������о���
ijѧϰС���������������о����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijѧϰС���������������о���
ijѧϰС���������������о����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com