
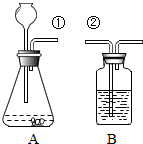
 ij����С�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�����װ��B��֤��������̼��ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���
ij����С�鰴��ͼ��ʾװ����ȡ������̼�������������̼�����ʣ�����װ��B��֤��������̼��ˮ��Ӧ����̼�ᣬB�г�������ˮ�⣬��Ӧ������Լ���| ʵ�� | ��1�� | ��2�� | ��3�� | ��4�� |
| ������Ʒ������/g | 5 | 10 | 15 | 20 |
| ����CO2������/g | 1.54 | 3.08 | 4.4 | m |
| ʯ��ʯ��̼��Ƶ����� |
| ʯ��ʯ������ |
| 100 |
| 44 |
| 5g��x |
| 1.54g |
| 100 |
| 44 |
| 5g��x |
| 1.54g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
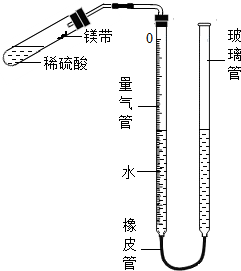 ij����С��ͬѧ��ͼװ�ã��̶�װ��δ�������ⶨ�������ʵ�þ����þ���ʵ�������������������Ӵ����������壩��ʵ������ǣ�
ij����С��ͬѧ��ͼװ�ã��̶�װ��δ�������ⶨ�������ʵ�þ����þ���ʵ�������������������Ӵ����������壩��ʵ������ǣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��ȫ���п����������ר����������Ʊ� ���ͣ�̽����
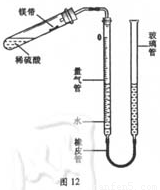
��2011�긣�����ݣ�17�⣩ij����С��ͬѧ��ͼ12װ�ã��̶�װ��δ�������ⶨ�������ʵ�þ����þ���ʵ�������������������Ӵ����������壩��ʵ������ǣ�
��ȡһ��þ����Ʒ��ȷ�Ƶ�������Ϊ0.030g��
������������װˮ�����ڿ̶ȡ�0����λ�á���ͼ12��ʾ��δװҩƷ��װ���������
�ۼ��װ�������ԡ�
�����Թ��м���������ϡ���ᣬ����б�Թܣ�����ˮʪ���þ��С�������Թܱ��ϣ�������Ƥ����
�ݵ���������Һ�棬ʹ���ߵ�Һ�汣��ͬһˮƽ����¼��������Һ��λ�á�
�ް��Թܵײ���Ϊ̧�ߣ�ʹþ����ϡ����Ӵ���ȫ��Ӧ��
�ߴ���ȴ�����º��ٴμ�¼��������Һ��λ�á�
����������������ӵ��������Ϊ23.96mL��
��֪��ͬ��ͬѹ�£���ͬ�����Ϻ�������ڻ��ǰ���������֮�͡�
��ش��������⣺
��1��д��þ��ϡ���ᷴӦ�Ļ�ѧ����ʽ ��
��2���ܷ���������ƽ����0.030gþ����Ʒ ������ܡ����ܡ���
��3�������ı��¶ȣ�����üķ������ͼ12��ʾװ�õ������ԣ�
��4�������㣬��ʵ���õ���������Ϊ0.0020g����þ���е���þ������������ ��
��5��ʵ����̢����ٴμ�¼�����ܵ�Һ��λ��ʱ���Թ��������������Ƿ��Ӱ��ʵ�����ս�� ����ǡ����������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡüɽ�������ر������о��꼶���£����л�ѧģ���Ծ��������棩 ���ͣ������
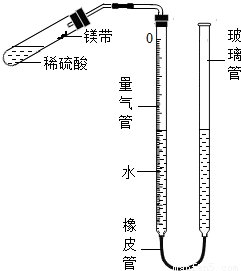
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������������ѧ���꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com