
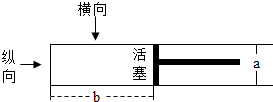
 ��ɫ������ɫ��dz�뵥λ���������������Լ��۲췽��������ĺ���йأ���ͼ����һ��������ֱ��Ϊa��ԲͲ���ܱղ��������г���һ�����Ļ���ɫ����Cl2����ʱ��������λ����ͼ��ʾ��
��ɫ������ɫ��dz�뵥λ���������������Լ��۲췽��������ĺ���йأ���ͼ����һ��������ֱ��Ϊa��ԲͲ���ܱղ��������г���һ�����Ļ���ɫ����Cl2����ʱ��������λ����ͼ��ʾ������ ��1��������ɫ������ɫ��dz�뵥λ����������������ϵ������ɣ�
��2��������ɫ������ɫ��dz�뵥λ���������������е����߹۲������
��3��������ɫ������ɫ��dz�뵥λ���������������е����߹۲������ɣ�
��� �⣺��1����a��b����Ӧ����۲�ʱ�������ɫ���
��2�������ֺ���۲죬�������������������������У��۲쵽��������ɫ����dz��
��3������������۲죬�����ƶ����������ƶ������У��۲쵽��������ɫ�����䣬
�ʴ�Ϊ����1������ ��2����dz ��3������
���� ���⿼�鷨��Ķ�����������⣬�ؼ��Ǹ�����ɫ������ɫ��dz�뵥λ����������������ϵ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ��ij�Լ�ƿ��ǩ�IJ������ݣ���ش��������⣺
��ͼ��ij�Լ�ƿ��ǩ�IJ������ݣ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ij��ɫ�����Ƕ�����̼����ȼ�ŵ�ľ�� | |
| B�� | ͨ����������ζ�ķ���������ں���ë��ά | |
| C�� | ϡ��Ũ����ʱ����ˮ����������ע��Ũ����������Ͻ��� | |
| D�� | ��pH��ֽ�ⶨ��Һ����ʱ���Ƚ�pH��ֽ��ˮʪ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�鲽�� | ���ͻ���� |
| ��1�������½ྻ�Ŀ���ͨ������������Һ����ͨ��Ũ���� | ͨ������������ҺĿ����Ϊ�˿����еĶ�����̼��ͨ��Ũ�����Ŀ����Ϊ�˳�ȥ�����е�ˮ������ |
| ��2��������ͨ�����ȵ�ͭ�� | ��ȥ�����е������� |
| ��3���ռ����壬���ⶨ��������ܶ� | �ܶ�Ϊ1.2572g/�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com