
 ������ճ������ũҵ�����벻��ˮ������Һ���ش�
������ճ������ũҵ�����벻��ˮ������Һ���ش����� ��1�����ݹ��˲����õ�������������
��2��������ˮ��Ӳˮ�ĸ�����з�����
��3��������Ծ����̶Ƚϸߵ���������н��
��4�����ݵ��ˮʵ��������з�����
��5�����ݹ�����ܽ�����߿��ԣ��ٲ��ij������һ���¶��µ��ܽ�ȣ��Ӷ�ȷ�����ʵ��ܽ��ԣ��ڱȽϲ�ͬ������ͬһ�¶��µ��ܽ�ȴ�С���Ӷ��жϱ�����Һ�����ʵ����������Ĵ�С�����ж����ʵ��ܽ�����¶ȱ仯�ı仯����ȣ�
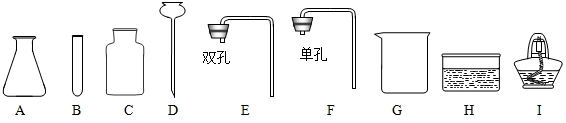
��� �⣺��1��������Ҫ�õ��������д���Ȧ������̨���ձ���©���Ͳ�������
��2�����н϶�����Ըơ�þ�������ˮ����Ӳˮ��
��3����Ծ����̶Ƚϸߵ�����������õ���ˮ������ˮ��
��4�����ˮʱ�������������IJ������ڲ����������������������ȼ�ŵ�ľ�����������˲����ܲ��������壬������ľ��ȼ�ո�����
��5������������ʣ������������¶�Խ�ߣ�ʣ�����Խ�࣬�����ܽ�������¶����߶���С�ģ�
��t1��ʱ��ʣ����嶼��10g��˵���ܽ���20g���ס��ҵ��ܽ�Ⱦ�Ϊ20g��
���¶���t2�潵�͵�t1��ʱ��ʢ�м���Һ���ձ��еĹ���������10g��С��15g��
��c��ʣ��������٣�a�д�֮��b��ʣ�������࣬����a��b��c���������ʵ����������ɴ�С��˳���ǣ�cab��
�ʴ�Ϊ����1��©������2��Ӳˮ����3��D����4��ľ��ȼ�ո�������5���ټ�С����20����С�ڣ� ��cab��
���� �����ѶȲ��Ǻܴ���Ҫ�����˹�����ܽ����������ʾ�����壬�����ݹ�����ܽ�������������ص����⣬�Ӷ������������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��
�� ��������ͬ���ǣ�������
��������ͬ���ǣ�������| A�� | ������ | B�� | ������ | C�� | ���Ӳ��� | D�� | ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H3BO3�� H��B��OΪ����Ԫ�ص�ԭ�Ӹ�����Ϊ3��1��3 | |
| B�� | H3BO3��H��B��OΪ����Ԫ�ص���������3��11��48 | |
| C�� | H3BO3��BԪ�ص�����������$\frac{11}{1+11+16}$��100% | |
| D�� | �����������������մ��Ƥ���ϣ�Ӧ�����ô�����ˮ��ϴ��Ȼ��Ϳ��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������-������ | B�� | ����-����� | C�� | ����þ-���� | D�� | ̼����-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | X�����Խ�ǿ | B�� | Yһ����ˮ | ||
| C�� | Z��һ���Ǽ���Һ | D�� | ��ϡ�����еμ�Z����ʹ��Һ�к� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com