
| ��Ӧʱ�� | t0 | t1 | t2 | t3 | t4 |
| �ձ���ҩƷ����/g | 25.7 | 25.6 | 25.5 | 25.5 | m |
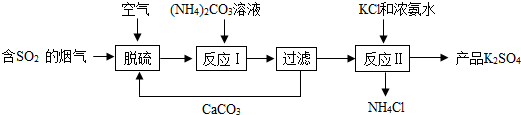
���� ��1��������������������ǣ���2�������������Ӱ�����ؿ��ǣ���3���ٸ��ݱ��������жϷ�Ӧ�Ƿ���ȫ��Ӧ���ڸ��������ļ�������������������������������������������μӷ�Ӧ�������������ٸ���ͨ�뵪�������ûش��⿼�ǣ��ڱ�����Ҫ������b�е��Լ������������������ɶ�����̼������������b���Լ����������ն�����̼�ģ����Կ����ü���Һ���۸���b�е��Լ�������3.3g�Ƕ�����̼�����������ݶ�����̼�����������������������������������������������Ʒ�������ɣ�
��� �⣺��1������������������������ˮ��ͬ���õĽ����
��2�������ηֵ��������ܴٽ������⣬�ӿ������ٶȣ�
��3���ٸ��������ļ�������������������������ͼʾ���ݿ�֪����t2�Ѿ���Ӧ���ˣ�����t4�ձ���ҩƷ�����Dz���ģ�����25.5�ˣ�
�ڸ��������ļ�����������������������25.7g-25.5g=0.2g����Ҫ����0.2g������Ҫ��������ΪX��
Fe+2HCl�TFeCl2+H2��
56 2
X 0.2g
���ݣ�$\frac{56}{x}=\frac{2}{0.2g}$
���X=5.6g��
�ټ���ǰҪ����ͨ��һ��ʱ�䵪����Ϊ���ž�װ���ڵĿ����������������������ľ̿��Ӧ�����ɶ�����̼�������ֹͣ���Ⱥ�Ҫ����ͨ��һ��ʱ�䵪������Ϊ�˰ѷ�Ӧ���ɵĶ�����̼������bװ���ڣ������в��ֶ�����̼������װ���ڣ�ʹ������̼�����٣��������������������٣����ƫС��
�ڱ�����Ҫ������b�е��Լ������������������ɶ�����̼�����������ݶ�����̼�����������������������������b���Լ����������ն�����̼�ģ����Կ����ü���Һ��������������Һ����Ӧ�����������ƺͶ�����̼����������̼���ƺ�ˮ���ù۲취��ƽ���ɣ�
�۸���b�е��Լ�������3.3g�Ƕ�����̼����������Ҫ����3.3g������̼��Ҫ�μӷ�Ӧ��������������ΪY��
2Fe2O3+3C$\frac{\underline{\;����\;}}{\;}$4Fe+3CO2
320 132
Y 3.3g
���ݣ�$\frac{320}{y}=\frac{132}{3.3g}$
���Y=8g���ѱ��ʵġ�ȡůƬ����Ʒ��Fe2O3������������$\frac{8g}{10g}��100%$=80%��
�ʴ�Ϊ����1��������ˮ����2���ڣ���3����25.5����5.6�����ž�װ���ڵĿ�����ƫС�����������ƣ�CO2+2NaOH�TNa2CO3+H2O������Ʒ��Fe2O3����������80%��
���� �����ؼ���֪���������ᷴӦʱ���������ļ������������������������ٸ��������������������������̼����������Ӧʱ����b�����������������ɵĶ�����̼�����������ݶ�����̼���������������������������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 84��85��79 | B�� | 1��1��1 | C�� | 23��24��18 | D�� | 1��1��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 С���ǵ���ѧϰ�������˳���Ӧ��ʱ����ʦ��˵��һ�㲻����K��Ca��Na�Ȼ��ý���������Һ�����û���Ӧ����Ϊ��Щ������ֱ�Ӻ�ˮ������Ӧ���ɶ�Ӧ�ļ��������С�������������£�
С���ǵ���ѧϰ�������˳���Ӧ��ʱ����ʦ��˵��һ�㲻����K��Ca��Na�Ȼ��ý���������Һ�����û���Ӧ����Ϊ��Щ������ֱ�Ӻ�ˮ������Ӧ���ɶ�Ӧ�ļ��������С�������������£�| ʵ�鷽�� | ʵ������ | |
| �� | ������������ɫ��ĩ | ��ɫ��ĩ�ɱ�����ȫ������ |
| �� | ȡ������ɫ��ĩ����ϡ������ | ������ |
| ʵ�鷽�� | ʵ������ | ���ۻ�ѧ����ʽ |
| 1��ȡʵ���ĺ�ɫ��ĩ������������A��Һ������ʹ��Ӧ��֣� | ��ɫ���ʱ����к�ɫ���ʣ���Һ��ɫ���ֽ�dz����ɫ���ձ��ײ����н϶��ɫ���� | Fe+CuSO4=FeSO4+Cu |
| 2�����ˡ�ϴ�ӡ�������ô������� | ��ɫ���ʿɱ�����ȫ�����������º�ɫ���� | �������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH | B�� | MgCl2 | C�� | Na2CO3 | D�� | CaO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʳ�︯�� | B�� | �̻���ը | C�� | ������ | D�� | ʪ�·����� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com