
��5�֣������³��ڵ�����������Ҫ�����ĸ��������г�������Ʒ���������ʲ�����ˮҲ�����ᷴӦ����ʵ��С��ͬѧΪ�ⶨ�䴿�ȣ�
����������ʵ�飬ʵ���������±���ʾ����ش��������⣺
| | ��1�� | ��2�� | ��3�� |
| ��Ʒ������g�� | 25 | 30 | 25 |
| ����������g�� | 200 | 100 | 100 |
| ������Һ������g�� | 216 | 116 | 116 |
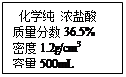
��1��Fe2O3+6HCl===2FeCl3+3H2O ��2��=
��3��20% ��4��200 ��5��404t
���������������1�����������Ҫ�ɷ����������������ᷴӦ�Ļ�ѧ����ʽΪ��Fe2O3 + 6HCl = 2FeCl3 + 3H2O
��2�� ��������ʵ�����ݷ��������γ�������Ʒ�е�������ǡ����100g���ᷴӦ��ȫ������������Һ����������֪25g��������Ʒ�е�������������Ϊ16g�����Ը��ݷ���ʽ��Fe2O3+6HCl==2FeCl3+3H2O��Fe2O3��HCl��������ϵ160:219�����Ե�2�βμӷ�Ӧ����������������(X)�ı���ʽ��160:219=16g��x
��3�� ������������������γ�������Ʒ�е�������ǡ����100g���ᷴӦ��ȫ���ɸ��ݷ���ʽ��Fe2O3+6HCl==2FeCl3+3H2O����������ʵ�����
�⣺��FeCl3������Ϊx
Fe2O3+6HCl==2FeCl3+3H2O
325
16g x
160��325=16g��x x=32��5g
��Һ������=116g+46��5g=162��5g
�������õ���Һ�����ʵ���������=32��5g/162��5g��100%=20%
��4��ͨ���ڶ��ʵķ�����100g��������������Ϊ21��9g, ����ʵ�������������400g������������=21��9g��4=87��6g������������Ũ��������Ϊv,����ʽΪ��V��1��2g/mL��36��5%=87��6g,V=200g
��5����Ϊ25g��������Ʒ�е�������������Ϊ16g ������500t�ÿ�ʯ��������������=320t,����������=500t-320t=180t������������=320t��112/160��100%=224t�����Ժ����ʵ���������Ϊ=180t+224t=404t
���㣺ʵ�����ݷ����ʹ��������ݻ�ѧ����ʽ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Сǿȥ�̵����һ�������װ˵������ͼ��Ϊ�ⶨ�ô�����̼���ƺ����Ƿ���ϱ�ǩҪ������ȡ�˴�����Ʒ11g��ȫ���ܽ���50gˮ�У�������ϡ����64.4gʱ��ǡ����ȫ��Ӧ��������Һ������Ϊ121g������
��1����Ӧ�ų���CO2������Ϊ g��
��2��������Ʒ��̼���Ƶ�������
��3��������һ�μ�⣬���㲢�ж���Ʒ��̼���Ƶĺ����Ƿ���ϱ�ǩҪ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��50g Na2CO3��Һ����μ���һ����������������CaCl2��Һ��ʵ������У����ɳ������������CaCl2��Һ��������ϵ����ͼ��ʾ���Լ��㣺
��1��ǡ����ȫ��Ӧʱ�����ɳ���������Ϊ______________g��
��2������ǡ����ȫ��Ӧʱ��������Һ�����ʵ�����������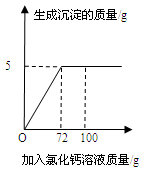
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
100��ijŨ�ȵ�����ǡ����13�˵�п��ȫ��Ӧ������㣺
��1�����������������������ȷ��0.1g����
��2������1���Ľ������ͼ�У�
��3����Ӧ��������Һ�����ʵ�����������д��������̣������ȷ��0.1%����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ش�����������йص����⣺
��1������Ƭ�봿��Ƭ��̻�ʱ������Ƭ��������»��ۣ�˵���� ����Ӳ�ȴ�
��2��6.5g��������ϡ���ᷴӦ����ѧ����ʽΪZn+H2SO4�TZnSO4+H2�����������Ͽ��Ƶ�����������
�� ��g��
��3��ʹ�����и����Լ�������֤Fe��Cu��Ag���˳������� ��
A��Ag��FeSO4��Һ��CuSO4��Һ
B��Fe��Cu��AgNO3��Һ
C��Fe��Ag��CuSO4��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��7�֣���ȤС���ͬѧΪ�ⶨijһ��ͭ�Ͻ��к�����������������6g�úϽ��ĩ��Ʒ������������������Ϊ10%������ͭ��Һ160g�У�����ǡ����ȫ��Ӧ��ͬʱΪ�˳��������Դ�����Է�Ӧ������ʽ��л��մ�������������ͼʾ���㣺
��1���úϽ���Ʒ�к�������������������������ȷ��0.1%��
��2�����Ĺ���ͭ������aΪ����g��
��3��������Һ�м������gˮ���ܵõ�5%������������Һ�����ڻ��ܵ�Ӫ��Һ��
����2����3���м�������ȷ��0.1g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(7��)ˮ������֮Դ����������ϧˮ��Դ��ÿ����������Ρ�
��1����Ȼ���е�ˮ���Ǵ�ˮ�������ó����� ������������ȷ������Ծ���ˮ��
��2��ˮ���������Ƹ�����Һ��������������ˮ����Һ�¶��������ߵ��� ��
| A���������� | B������� | C������� | D��Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��˾�������Ĵ����Ʒ�о����ֻ�����Ȼ������ʡ�Ϊ�ⶨ��Ʒ��̼���Ƶ�����������20��ʱ����ȡ�ò�Ʒ��Ʒ26��5g�����뵽ʢ��һ������ϡ������ձ��У�̼������ϡ����ǡ����ȫ��Ӧ��������ȫ�ݳ����õ�������NaCl��Һ����Ӧ�����þ�����������ձ��ڻ�����������m���뷴Ӧʱ�䣨t����ϵ����ͼ��ʾ��
��1������CO2��������
��2����Ӧ��������Һ��NaCl������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ݻ�ѧ����ʽ���㣺��ȫ�ֽ�340g������������Ϊ10%�Ĺ���������Һ�еĹ������⣨H2O2����������������������
| A��16g | B��160g | C��32g | D��1.6g |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com