
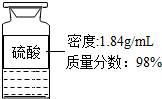
 ij�Ƽ�С��Ϊ����֤����������������NaOH��Һ�Ƿ�ﵽŨ�ȱ�����NaOH 35%��48%�������������²�����
ij�Ƽ�С��Ϊ����֤����������������NaOH��Һ�Ƿ�ﵽŨ�ȱ�����NaOH 35%��48%�������������²��������� ��1��Ũ���᳨�ڷ��û���ˮ��������������������С��
��2��������pH��ֽ�ⶨ��ҺpH�ķ���������ɣ�
��3��������Ŀ�е���������ݺͻ�ѧ����ʽ�����м��㣮
��� �⣺��1��Ũ���᳨�ڷ��û���ˮ��������������������С��
��2����pH��ֽ�ⶨδ֪��Һ��pHʱ����ȷ�IJ�������Ϊ�ڲ���Ƭ��״ɰ��ϣ��ò�����պȡ��������Һ���ڸ����pH��ֽ�ϣ������ɫ���Աȣ����ɲ����Һ��pH��
��3����֪�����кͲⶨ����pH=7ʱ������160g24.5%��ϡ���ᣬ�����100gNaOH��Һ�е�������������Ϊx����
2NaOH+H2SO4�TNa2SO4+2H2O
80 98
x 160g��24.5%
��$\frac{80}{x}$=$\frac{98}{160g��24.5%}$��
���x=32g��
������������������Ϊ$\frac{32g}{100g}$��100%=32%��35%��48%����û�дﵽ��Ũ�ȱ���
�ʴ�Ϊ����1����С
��2���ýྻ�IJ�����պȡ����Һ���ε�pH��ֽ�ϣ�Ȼ��ͱ���ɫ������
��3��û�дﵽ��Ũ�ȱ���
���� ������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������


 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ͼA��B��C���ֹ�����ܽ�����ش�
������ͼA��B��C���ֹ�����ܽ�����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ���ڿ�����ȼ�����ɶ�����̼��ˮ | |
| B�� | ���ڿ�����ȼ�ղ�����ɫ���� | |
| C�� | ��˿�ڿ����о���ȼ�ա��������� | |
| D�� | ľ̿ȼ������ʹ����ʯ��ˮ����ǵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ���ӽṹʾ��ͼ
��ͼ���ӽṹʾ��ͼ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com