
 ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã�����ͼ������ѧ�Ƽ������߰�ȼ�����нϴ�ķ����ѽ�ɺ��������������ĸ�̼ԭ�ӵȵ�С���ӣ�Ȼ������Ǽӹ��Ƴɸ��ֲ�Ʒ�������ϡ��ϳ���ά���ϳ���ҩ�ũҩ��ըҩ�����ʵȵȣ�
ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã�����ͼ������ѧ�Ƽ������߰�ȼ�����нϴ�ķ����ѽ�ɺ��������������ĸ�̼ԭ�ӵȵ�С���ӣ�Ȼ������Ǽӹ��Ƴɸ��ֲ�Ʒ�������ϡ��ϳ���ά���ϳ���ҩ�ũҩ��ըҩ�����ʵȵȣ�
 2CO2+3H2O��
2CO2+3H2O��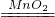 2H2O+O2����
2H2O+O2����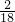 ��100%=
��100%= g�����
g����� ��
��

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
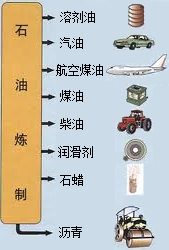 �й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����
�й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����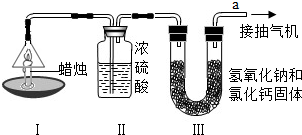
| ���� | װ�â� | װ�â� | |
| ��Ӧǰ������/g | 15.8 | 182.3 | 212.2 |
| ��Ӧ�������/g | 14.4 | 184.1 | 216.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã�����ͼ������ѧ�Ƽ������߰�ȼ�����нϴ�ķ����ѽ�ɺ��������������ĸ�̼ԭ�ӵȵ�С���ӣ�Ȼ������Ǽӹ��Ƴɸ��ֲ�Ʒ�������ϡ��ϳ���ά���ϳ���ҩ�ũҩ��ըҩ�����ʵȵȣ�
ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã�����ͼ������ѧ�Ƽ������߰�ȼ�����нϴ�ķ����ѽ�ɺ��������������ĸ�̼ԭ�ӵȵ�С���ӣ�Ȼ������Ǽӹ��Ƴɸ��ֲ�Ʒ�������ϡ��ϳ���ά���ϳ���ҩ�ũҩ��ըҩ�����ʵȵȣ�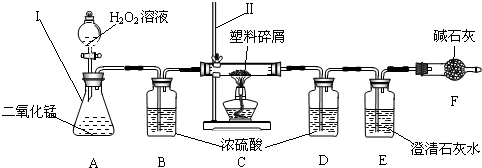
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 �й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����
�й�ʯ����Ȼ�����Ź�˾�����������ڲ�����̲���������ִ�����ģ��ʮ�ڶֵĴ�����--�����ϱ���������ǿ�ҹ���Դ��ȫ��Ӧ�ı�������������Ҫ���壮ʯ������Ҫ����̼��������Ԫ�أ�����ʯ���и��ɷֵķе㲻ͬ�������Ƿ��루��Ϊ�����ɵõ���ͬ�IJ�Ʒ��ʹʯ�͵õ��ۺ����ã���ͼ����
| ||
| ||

| ���� | װ�â� | װ�â� | |
| ��Ӧǰ������/g | 15.8 | 182.3 | 212.2 |
| ��Ӧ�������/g | 14.4 | 184.1 | 216.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008�꽭��ʡ�Ͼ����������п���ѧ��ģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com