
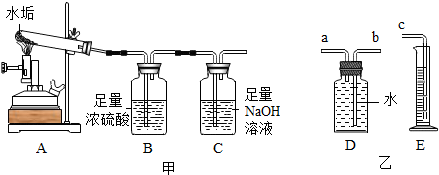
���� ��1��Ũ���������ˮ�ԡ���ˮ�ԡ�ǿ�����ԣ�
��2�����ݻ�ѧ����ʽ���Խ�����ط���ļ��㣻
��3������b����Ӧ��c���������ſ��Խ�ˮ�ŵ���Ͳ�У���Ͳ���ռ�����ˮ���������Ϊ����D�Ķ�����̼�������
��� �⣺��1��Ũ���������ˮ�ԣ�Ũ�����ܹ��������շ�Ӧ�в�����ˮ������
������շ�Ӧ�в�����ˮ������
��2��Cװ��������������Һ������1.76g��˵��̼��Ʒֽ�������1.76g������̼��
��̼��Ƶ�����ΪX��
CaCO3$\frac{\underline{\;����\;}}{\;}$CaO+CO2��
100 44
X 1.76g
$\frac{100}{X}$=$\frac{44}{1.76g}$
X=4g��
��ˮ������̼��Ƶ���������Ϊ��$\frac{4g}{5g}$��100%=80%��
���80%��
��3��������b����Ӧ��c���������ſ��Խ�ˮ�ŵ���Ͳ�У��������������ʴ���
����Ҫ����װ����ˮ�Ϸ���һ����Ĥ����ֹ������̼����ˮ������ȷ��
����Ͳ���ռ�����ˮ���������Ϊ����D�Ķ�����̼�������Dװ���в�����Ҫʢ��ˮ���ʴ���
�ܶ���ʱӦ����D��E��װ����Һ����ƽ��ʹ����ѹ����ȣ�Ϊ�˽�ȷ������ȷ��
����ڢ�
���� ��Ҫ������ѧ�����������ơ�Ũ��������ʵ���ʶ����ij�����ʵ���������ʱ��ע�ⲻҪ©����100%����������ʱ�������Եģ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
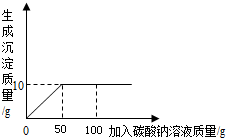 Ϊ�ⶨ���Ȼ��ƺ��Ȼ�����ɵĹ�����Ʒ���Ȼ��Ƶĺ�����ijͬѧ����������ʵ�飺ȡ20g������Ʒ��ȫ������100gˮ�У������õĻ����Һ�еμ�̼������Һ����¼����������ͼ��ʾ�����ߣ�
Ϊ�ⶨ���Ȼ��ƺ��Ȼ�����ɵĹ�����Ʒ���Ȼ��Ƶĺ�����ijͬѧ����������ʵ�飺ȡ20g������Ʒ��ȫ������100gˮ�У������õĻ����Һ�еμ�̼������Һ����¼����������ͼ��ʾ�����ߣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
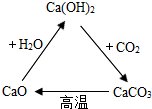 ��ͼ�ǹ��ɵļ��ֺ��ƻ������֪ʶ����Ȧ�����ݸ�ͼ�ش�
��ͼ�ǹ��ɵļ��ֺ��ƻ������֪ʶ����Ȧ�����ݸ�ͼ�ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
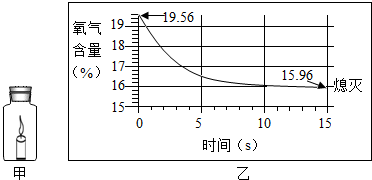
| A�� | ����ȼ��ǰװ����ֻ������ | |
| B�� | ����Ϩ���ƿ��ֻʣ������̼���� | |
| C�� | ������װ�����������������ϼ��� | |
| D�� | ��������С��һ��ֵʱ��������ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 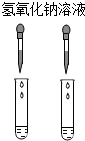 | B�� | 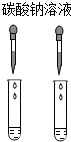 | C�� | 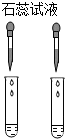 | D�� | 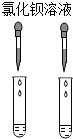 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | +1�� | B�� | +2�� | C�� | +3�� | D�� | ��ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ijѧϰС���������������о���
ijѧϰС���������������о���| ʱ��/h | 1 | 1.5 | 4 | 8 | 12 | 24 | 48 | 60 | |
| ��ˮ ����/g | Ũ���� | 1.6 | 2.2 | 5.2 | 10.3 | 14.0 | 20.9 | 29.2 | 32.1 |
| ϡ���� | 1.2 | 1.5 | 3.5 | 5.9 | 8.1 | 12.9 | 19.5 | 21.0 | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com