
| 1 |
| 10 |
| 1 |
| 10 |
| 1 |
| 5 |
| 1 |
| 5 |
| 1 |
| 10 |


科目:初中化学 来源: 题型:
 学们对竹子中的气体成分展开了探究,测定其成分.
学们对竹子中的气体成分展开了探究,测定其成分.| 实验步骤 | 实验现象 | 实验结论及解释 |
| 向乙瓶气体中倒入,振荡 | 反应的化学方程式为: |
查看答案和解析>>
科目:初中化学 来源: 题型:阅读理解
 某同学在一次玩耍中,偶然发现竹子浸没水中后,在竹子上打一小洞,会看到水中有很多小气泡冒出.
某同学在一次玩耍中,偶然发现竹子浸没水中后,在竹子上打一小洞,会看到水中有很多小气泡冒出.
| ||
| ||
查看答案和解析>>
科目:初中化学 来源: 题型:
 我市地处皖南山区,竹子是我市的主要经济作物之一,也是同学们非常熟悉的一种植物.同学们对竹节中的气体成分展开了探究.
我市地处皖南山区,竹子是我市的主要经济作物之一,也是同学们非常熟悉的一种植物.同学们对竹节中的气体成分展开了探究.| 1 |
| 10 |
| ||
| ||
| 实验步骤 | 实验现象 | 结论及解释 |
| 向乙瓶中倒入澄清石灰水,振荡 | 澄清石灰水立刻变浑浊 | 反应的化学方程式为: CO2+Ca(OH)2=CaCO3↓+H2O CO2+Ca(OH)2=CaCO3↓+H2O |
查看答案和解析>>
科目:初中化学 来源:福建省连江县文笔中学2012届九年级上学期期中考试化学试题 题型:059
某同学在一次玩耍中,偶然发现竹子浸没水中后,在竹子上打一小洞,会看到水中有很多小气泡冒出.
提出问题:竹子中含有什么气体呢?
猜想与假设:该气体中可能含有氧气,也可能含有二氧化碳.
查阅资料:氧气可以支持可然物燃烧;二氧化碳能使澄清的石灰水变浑浊.
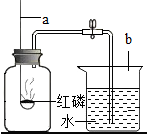
实验探究:(1)他先将竹子浸在水中,钻个小孔,看到一串串气泡冒出.然后采用________法收集到了甲、乙两瓶气体.
(2)将放有足量红磷的燃烧匙伸入甲瓶中(如图).用放大镜聚焦,发现红磷燃烧,瓶内充满了白烟,这说明竹子里的气体中含有________,该反应的文字表达式为________.该反应属于________反应,实验中,用放大镜聚焦对于红磷燃烧所起的作用是________.充分冷却后,将导管的一端放入水中,打开止水夹B,结果流入甲瓶中的水约占瓶子容积的1/10,说明了________.

(3)再往乙瓶中倒入适量澄清石灰水并振荡,发现石灰水变浑浊.说明竹子里的气体中含有________.
拓展与迁移:那么竹子里的气体含量与空气中的气体含量有什么区别呢?
(4)于是,他用空气也做了上述实验.相比较后得出的竹子中的气体和空气在组成上不同的结论是:________.
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com