
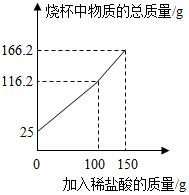
 �տ�ˮ�Ĵ���������һ��ܺ��ˮ����ˮ������Ҫ�ɷ���CaCO3��Mg��OH��2��Ϊ��Ū��ˮ����̼��Ƶ�����������ȡһ����������������Ʒ�����ձ��У����ձ��еμ�һ����������������ϡ���ᣬʵ�����ձ������ʵ�����������������������ϵ��ͼ��ʾ����
�տ�ˮ�Ĵ���������һ��ܺ��ˮ����ˮ������Ҫ�ɷ���CaCO3��Mg��OH��2��Ϊ��Ū��ˮ����̼��Ƶ�����������ȡһ����������������Ʒ�����ձ��У����ձ��еμ�һ����������������ϡ���ᣬʵ�����ձ������ʵ�����������������������ϵ��ͼ��ʾ�������� ���������غ㶨�ɿ�֪�������������ļ�������Ϊ�����˶�����̼�����Կ������������̼�����������ݶ�����̼�������Ͷ�Ӧ�Ļ�ѧ����ʽ����̼��Ƶ����������������Ӧ����������
��� �⣺��ͼ��֪������������Ϊ0ʱ�ձ������ʵ�����Ϊ25g��������Ʒ������Ϊ25g
���������غ㶨�ɿɵã����ɵĶ�����̼������Ϊ25g+150g-166.2g=8.8g
���ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 8.8g
$\frac{100}{44}$=$\frac{x}{8.8g}$
x=20g
��Ʒ��̼��Ƶ���������Ϊ$\frac{20g}{25g}$��100%=80%
�𣺣�1����ȡ��Ʒ��������25g��
��2�����ɶ�����̼�������� 8.8g��
��3����Ʒ��̼��Ƶ���������Ϊ80%��
���� ���ݻ�ѧ����ʽ����ʱ����һҪ��ȷ��д��ѧ����ʽ���ڶ�Ҫʹ����ȷ�����ݣ������������Ҫ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��Ӧǰ��ԭ�ӵ������������ | |
| B�� | ��Ϊ�л����̼Ԫ�ص���������Ϊ75% | |
| C�� | �÷�Ӧ�мס��Ҽ�������Ϊ1��1 | |
| D�� | �÷�Ӧ���û���Ӧ����Ӧ��̼Ԫ�ػ��ϼ۽��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ϡ��һ��������������������������Һ | |
| B�� |  �ں��������£������͵�NaCl��Һ��������ˮ | |
| C�� | 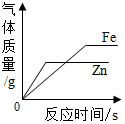 ��������п������������ϡ���ᷴӦ | |
| D�� | 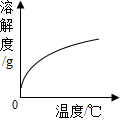 ��ʯ�ҵ��ܽ�����¶ȵĹ�ϵ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | +2 | B�� | +3 | C�� | +4 | D�� | +6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���շϾɽ��� | B�� | ����ʹ��һ����������Ʒ | ||
| C�� | �����ŷŹ�ҵ��ˮ��������ˮ | D�� | ʵ���ҷ��ﲻ���������ⶪ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ��ͼ��A��B��C�������ʵ��ܽ�����ߣ�����˵������ȷ���ǣ�������
��ͼ��A��B��C�������ʵ��ܽ�����ߣ�����˵������ȷ���ǣ�������| A�� | t1��ʱ��A��B��C�������ʵ��ܽ���ɴ�С��˳����C��B��A | |
| B�� | ��A�к�������Bʱ������ͨ����ȴ�ȱ�����Һ�ķ����ᴿA | |
| C�� | t2��ʱ��A��C�ı�����Һ�к�����ͬ���������� | |
| D�� | ��t2��ʱA��B��C�ı�����Һͬʱ������t1���������Һ�����ʵ����������ɴ�С��˳����B��C��A |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com