
����Ŀ��������и��⣺
(1)ʵ���ұ���FeCl2��Һʱ���������м�����������ԭ����__________________(�����ӷ���ʽ��ʾ)��
ijͬѧΪ����ijδ֪��Һ���Ƿ�ΪFeCl2��Һ����ȡ����ʵ�鷽��������֤����ȡδ֪��Һ���Թ���![]() ��Һ
��Һ![]() ��Һ��Ѫ��ɫ(֤������FeCl2)������Ϊ�˷���________(���������������)��������������θĽ�_______________________________________(�����������ʲ���)��
��Һ��Ѫ��ɫ(֤������FeCl2)������Ϊ�˷���________(���������������)��������������θĽ�_______________________________________(�����������ʲ���)��
(2)A��B��C��D��E�ֱ����������Ļ��������D��һ�ֺ��ɫ�����������Ӧ��ϵ��ͼ��ʾ��
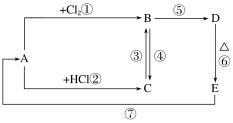
������ͼ��ʾ�仯����Ҫ��ش��������⣺
��д��A��E�Ļ�ѧʽ��
A________��E________��
��д�����м�����Ӧ�Ļ�ѧ����ʽ��
C��B��________________________________________________________________________��
B��D��________________________________________________________________________��
E��A��________________________________________________________________________��
��B�м���NaOH��Һ��������������________________________________��
���𰸡�(1)2Fe3++Fe===3Fe2+
������ �ȼ�KSCN��Һ���������ټ�����ˮ����Һ��ɺ�ɫ����֤��ԭ��Һ��Fe2+
(2)��Fe Fe2O3 ��2FeCl2+Cl2===2FeCl3(��������) FeCl3+3NaOH===Fe(OH)3��+3NaCl(��������)
Fe2O3+3CO![]() 2Fe+3CO2(��Fe2O3+2Al
2Fe+3CO2(��Fe2O3+2Al![]() 2Fe+Al2O3)
2Fe+Al2O3)
�����ְ�ɫ������Ѹ�ٱ�ɻ���ɫ�����ձ�ɺ��ɫ
��������(2)������Ĺؼ����ƶ�D����ΪDΪ���ɫ��������֪D��Fe(OH)3����E��Fe2O3��A��Fe��B��FeCl3��C��FeCl2��


 Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����Ҫ����գ�
(1)��S2��Fe2+��Fe3+��Mg2+��S��I��H+�У�ֻ�������Ե���____________��ֻ�л�ԭ�Ե���____________���������������л�ԭ�Ե���________��
(2)ijͬѧд������������ѧ����ʽ(δ��ƽ)��
��NO+HNO3��N2O3+H2O ��NH3+NO��HNO2+H2O ��N2O4+H2O��HNO3+HNO2
��������Ϊһ��������ʵ�ֵ���____________��
(3)��������������ԭ��Ӧ�У���������ǿ��������______________��
��2FeCl3+2KI![]() 2FeCl2+2KCl+I2
2FeCl2+2KCl+I2
��2FeCl2+Cl2![]() 2FeCl3
2FeCl3
��2KMnO4+16HCl(Ũ)![]() 2KCl+2MnCl2+5Cl2��+8H2O
2KCl+2MnCl2+5Cl2��+8H2O
��������Cl��I���棬Ϊ������I��Cl�����������������⣬��Ӧ��������Ӧ�е�__________����������
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ����ϸ������绷��֮��������ʽ����Ĺ��̡�A��B��C��D��ʾ������Һ���٢ڱ�ʾ�йص����ʡ����ͼ�ش�

��1��A��D�в������ڻ����ɷֵ��ǣ� �� ���� �� �����ھ������ϸ��ֱ������Ļ�����
��2��B��Һ�� ������ѭ������ ����C�С�B��C�ijɷ��������������Ҫ�IJ���� ��
��3��C����ѹ��С��Ҫ�� �ĺ����йأ������ȱ�������ȶ����������е� ��HPO42-�������йء�
��4������ڴ���O2����������뵽��֯ϸ����Ҫ ��ϵͳ��Э����ɣ�����ά����̬����Ҫ���ڻ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����֪AΪ�����Ľ������ʣ�������ͼ��ʾ�Ĺ�ϵ���ش��������⡣

(1)ȷ��A��B��C��D��E��F�Ļ�ѧʽ��AΪ________��BΪ________��CΪ________��DΪ________��EΪ________��FΪ________��
(2)д����Ӧ���Ļ�ѧ����ʽ�ͷ�Ӧ�����������ӷ���ʽ��
��____________________________________________________________��
��_____________________________________________________________��
��_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪһ����AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ����������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ�
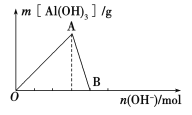
��ش��������⣺
(1)A��ʱ�Ѳμӷ�Ӧ��AlCl3��NaOH�����ʵ���֮��Ϊ________��
(2)AB����������ʾ�ķ�Ӧ�����ӷ���ʽΪ_________________________��
(3)��B�����ɵ���Һ��ͨ�������̼���ɹ۲쵽��������_______________________��
(4)����0.1 mol NH4Al(SO4)2����Һ����μ���5 mol��L1 NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����д̼�����ζ�������ݳ�������ɫ�������ٲ�������ʧ��������ͼ�л������ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵʾ��ͼ��
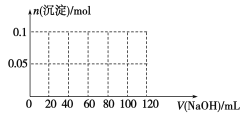
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��You do not have to count all the nuts. Just ________ how many there are.
A. aim B. change C. estimate D. join
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�� The general manager thought _______ of these problems before he made the final decision.
A. a good many B. a great deal
C. lots D. a plenty
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ������������е���Ƥ��˫��Ƥ֮�֣���һ�Ե�λ������A��a�����ơ�Ϊ�о��������Ŵ���ʽ��ijУ������ȤС��Ը�У��ͬѧ����ͥ��Ա��������ص��飬������±���
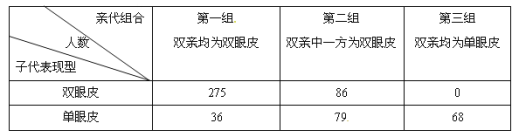
��ͼ�ش�
��1�����ݱ��е� �������������ȷ�ж� Ϊ������״��
��2���ڶ�����Ů����ij������ĸ��Ϊ����Ƥ�����Ľ�����Ϊ˫��Ƥ����ô
���������������Ŵ��Ļ���λ�� Ⱦɫ���ϡ�
����ij���Ļ������� �������ݸ���ij�Ļ����� ��
������ij�Ľ����һ������Ƥ�����Խ�飬��һ��˫��ƤŮ���ĸ���Ϊ ��
��3��д���ڶ���˫�Ļ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��You can't predict everything. Often things don't______as you expect.
A. run out
B. break out
C. work out
D. put out
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com