
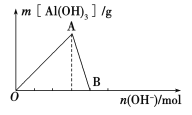
����Ŀ����ͼ��ʾΪһ����AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ����������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ�
��ش��������⣺
(1)A��ʱ�Ѳμӷ�Ӧ��AlCl3��NaOH�����ʵ���֮��Ϊ________��
(2)AB����������ʾ�ķ�Ӧ�����ӷ���ʽΪ_________________________��
(3)��B�����ɵ���Һ��ͨ�������̼���ɹ۲쵽��������_______________________��
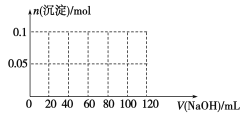
(4)����0.1 mol NH4Al(SO4)2����Һ����μ���5 mol��L1 NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����д̼�����ζ�������ݳ�������ɫ�������ٲ�������ʧ��������ͼ�л������ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵʾ��ͼ��
���𰸡�(1)13
(2)Al(OH)3+OH===![]() +2H2O
+2H2O
(3)���ɰ�ɫ����
(4)
��������(1)A������ﵽ���OA�η������ӷ�Ӧ����ʽΪ��Al3++3OH===Al(OH)3�����������ʵ���֮��Ϊ1��3��
(2)��������������������������AB�η������ӷ�Ӧ����ʽΪ��Al(OH)3+OH===![]() +2H2O��
+2H2O��
(3)B������ΪNaAlO2��ͨ�������̼������Ӧ��2![]() +CO2+3H2O===2Al(OH)3��+
+CO2+3H2O===2Al(OH)3��+![]() ������а�ɫ����������
������а�ɫ����������
(4)Al3+��OH������ǿ��![]() ������ȷ���Al3++OH===Al(OH)3��������������ʱ������NaOH�����Ϊ60 mL��Ȼ����
������ȷ���Al3++OH===Al(OH)3��������������ʱ������NaOH�����Ϊ60 mL��Ȼ����![]() +OH===NH3��H2O����ʱ�������������䣬�˽����ĵ�NaOH�����Ϊ20 mL�����������Al(OH)3+OH===
+OH===NH3��H2O����ʱ�������������䣬�˽����ĵ�NaOH�����Ϊ20 mL�����������Al(OH)3+OH===![]() +2H2O����ʱ���ĵ�NaOH�����Ϊ20 mL�����ͼ�������ʾ��
+2H2O����ʱ���ĵ�NaOH�����Ϊ20 mL�����ͼ�������ʾ��


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���±��Ǽ��ֳ���ȼ��(1 mol)��ȫȼ��ʱ�ų���������
���� | ̿��(C) | һ����̼(CO) | ����(H2) | ����(CH4) | �Ҵ�(C2H5OH) |
״̬ | ���� | ���� | ���� | ���� | Һ�� |
����(kJ) | 392.8 | 282.6 | 285.8 | 890.3 | 1 367 |
��1���������Ƕȷ�����Ŀǰ���ʺϼ�ͥʹ�õ���������ȼ����________��
��2��д����ʾ�ܵ�ú���е�һ����̼ȼ���ȵ��Ȼ�ѧ����ʽ_________________________��
��3�����ȼ��1 mol���и���ȼ�ϣ��ŷų�������̼����������________��
��4������ȼ�ϴ������ޣ�������ȼ�չ����л������Ⱦ��������Դ�������ķ�չս�ԣ��ҹ��������õ���ɫ��Դ�����ܡ�________�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���Ķ����ϣ��ش��������⡣
���ϣ����������Ƽ���Ա�о��õ�һ�����Ͳ��ϡ�����ĭ�������ǰѷ��ݼ��ӵ��������Ͻ����Ƴɵģ����ŵ���Ӳ�ȸߣ��ܶ�С(ԼΪ0.16��0.5 g/cm3)����ľ�Ļ��ᣬ�ɸ���ˮ�棬���кܴ���ԣ��Ҹ��������£���һ�����õĽ������Ϻ����ʲ��ϣ�������ɴ�����Ͷ���г���
(1)���й�����ĭ����˵���������________��
A����ĭ��������������ĭ
B����ĭ����һ�ֺϽ�
C����ĭ����һ�����ʵĽ������Ϻ����ʲ���
D����ĭ�������ڷɻ�����
(2)���Ƴ�������ʳƷ��װ��������������һ����________��
A���������� B����չ��
C�������� D��������
(3)���ڿ����лᱻ��������һ�����ܵ�����Ĥ(������)�������𱣻����ã����������Ĥ(������)����ǿ���ǿ����ܽ⣬��д����
�����ᷴӦ�����ӷ���ʽ��__________________________________________��
������������Һ��Ӧ�����ӷ���ʽ��__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ijУ��ѧ��ȤС������ͼ��ʾ���̳�ȥAlCl3�к��е�Mg2+��K+�������Ӳ������ܼ���AlCl3����ʧ����ش��������⣺
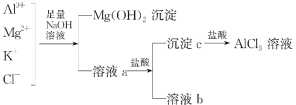
(1)д��������м�����������������Һʱ����Һ�з�����Ӧ�����ӷ���ʽ��_______________________��
(2)����������Һ�ܷ��ð�ˮ���棬Ϊʲô��___________________________��
(3)��Һa�д��ڵ�������________________������Һa�м�������ʱ��������������Ϊʲô��__________________________________��Ϊ�ˣ��Ľ�������___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��������и��⣺
(1)ʵ���ұ���FeCl2��Һʱ���������м�����������ԭ����__________________(�����ӷ���ʽ��ʾ)��
ijͬѧΪ����ijδ֪��Һ���Ƿ�ΪFeCl2��Һ����ȡ����ʵ�鷽��������֤����ȡδ֪��Һ���Թ���![]() ��Һ
��Һ![]() ��Һ��Ѫ��ɫ(֤������FeCl2)������Ϊ�˷���________(���������������)��������������θĽ�_______________________________________(�����������ʲ���)��
��Һ��Ѫ��ɫ(֤������FeCl2)������Ϊ�˷���________(���������������)��������������θĽ�_______________________________________(�����������ʲ���)��
(2)A��B��C��D��E�ֱ����������Ļ��������D��һ�ֺ��ɫ�����������Ӧ��ϵ��ͼ��ʾ��
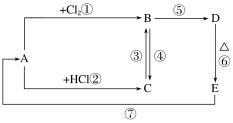
������ͼ��ʾ�仯����Ҫ��ش��������⣺
��д��A��E�Ļ�ѧʽ��
A________��E________��
��д�����м�����Ӧ�Ļ�ѧ����ʽ��
C��B��________________________________________________________________________��
B��D��________________________________________________________________________��
E��A��________________________________________________________________________��
��B�м���NaOH��Һ��������������________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�з����ķ�Ӧ��ijУ��ѧ��ȤС��ͬѧ�������ͼ��ʾ��ʵ��װ�á�
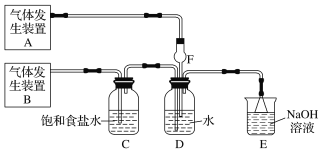
(1)�û�ѧ��ȤС���ͬѧΪ��ȡCl2��SO2���壬�ֲ���Na2SO3��70%������Ϊԭ����ȡSO2������MnO2��Ũ����(12 mol��L1)Ϊԭ����ȡCl2���ڴ�ʵ���У�F������������________������װ��BӦѡ����������װ���е�________(�����)��

(2)Dװ������Ҫ��Ӧ�����ӷ���ʽΪ_________________________________��
(3)Ϊ��֤ͨ��Dװ���е�������Cl2��������SO2��������ȤС���ͬѧ���������Լ���
���Ȼ�����Һ ���Ȼ�������Һ �����軯����Һ �����Ը��������Һ
��Cl2������ȡ����D����Һ�μ���ʢ��________(ѡ��һ�����)�Լ����Թ��ڣ��ټ���________(ѡ��һ�����)�Լ���������������______________________________________��
��SO2������ȡ����D����Һ�μ���ʢ��________(ѡ��һ�����)�Լ����Թ��ڣ�������������_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����Ӭ��Ⱥ�з���һֻ����ͻ�����Ӭ����Ұ����������������Ӭ������F1��Ϊ��������F1���������䣬F2�������۴�Ӭ�U��������Ӭ�U���۴�Ӭ�U������Ӭ��3�U3�U1�U1��
��1���������������״�Ļ���λ�� Ⱦɫ���ϣ�������״�� ��F2�����۹�Ӭһ��ϸ��������� �����ۻ���
��2����������ȱ��һ��4��Ⱦɫ��������۴��ϴ�Ӭ���������Ӿ������������۴�����Ӭ������̽�����ۻ����Ƿ���4��Ⱦɫ���ϡ����ӽ�������� ��˵������������4��Ⱦɫ���ϣ����ӽ�������� ��˵������������4��Ⱦɫ���ϡ�
��3����Ӭ�ĺ���������Ϊ���ԣ��ֱ�����λ����w������������Ҫͨ��һ���ӽ��������ж����Ի����Ƿ�λ��XȾɫ������Ӧ��ѡ�����ױ�Ϊ ����������λ��XȾɫ���ϣ���д�������ױ��ӽ��������Ӵ����Ŵ�ͼ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�����ҹ�����ij���������������ͷֲ���סլ��¥���ֲ�ʾ��ͼ���ش��������⡣
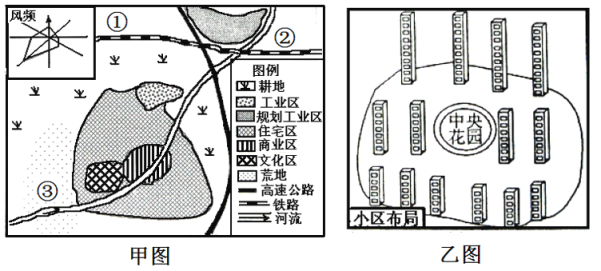
��1���õؼƻ������й�ҵǨ���滮��ҵ�����Է���ԭ��
��2��������������ѡ��һ�����ָ�סլ������ѡ����ѵص㲢���������ɡ�
��3��סլ���ڲ���¥��������ʲô�ص㣿������䲼�ֵ����ơ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com