
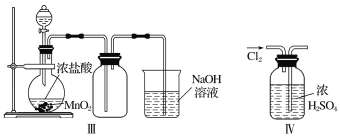
����Ŀ��Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�з����ķ�Ӧ��ijУ��ѧ��ȤС��ͬѧ�������ͼ��ʾ��ʵ��װ�á�
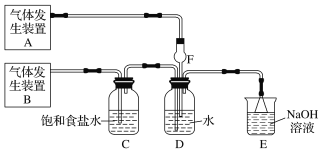
(1)�û�ѧ��ȤС���ͬѧΪ��ȡCl2��SO2���壬�ֲ���Na2SO3��70%������Ϊԭ����ȡSO2������MnO2��Ũ����(12 mol��L1)Ϊԭ����ȡCl2���ڴ�ʵ���У�F������������________������װ��BӦѡ����������װ���е�________(�����)��

(2)Dװ������Ҫ��Ӧ�����ӷ���ʽΪ_________________________________��
(3)Ϊ��֤ͨ��Dװ���е�������Cl2��������SO2��������ȤС���ͬѧ���������Լ���
���Ȼ�����Һ ���Ȼ�������Һ �����軯����Һ �����Ը��������Һ
��Cl2������ȡ����D����Һ�μ���ʢ��________(ѡ��һ�����)�Լ����Թ��ڣ��ټ���________(ѡ��һ�����)�Լ���������������______________________________________��
��SO2������ȡ����D����Һ�μ���ʢ��________(ѡ��һ�����)�Լ����Թ��ڣ�������������_______________________________________________________________��
���𰸡�(1)������ ��
(2)Cl2+SO2+2H2O===4H++![]() +2Cl
+2Cl
(3)�� �� ��Һ�ʺ�ɫ �� �Ϻ�ɫ��Ϊ��ɫ
��������(1)��ʵ��װ�ÿ�֪�����巢��װ��B�����������ñ���ʳ��ˮ���г��Ӿ�������֪Bװ��Ϊ��ȡCl2װ�ã���Aװ��Ϊ��ȡSO2��װ�ã���SO2������ˮ����F����������Ϊ����������ȡCl2�����Լ�ΪMnO2��Ũ���ᣬ���ڹ̡�Һ��ϼ�����ȡ���壬��Ӧѡ��װ��Ϊ����װ��B��
(2)��Cl2��SO2ͬʱͨ��ˮ��ʱ��Cl2��SO2��������H2SO4��Cl2����ԭΪHCl��
(3)��Cl2����������D����Һ�г���H2SO4��HCl�⣬������ʣ��Cl2��HClO������ǿ�����ԣ��ɽ�Fe2+����ΪFe3+���ʿ�ѡ���ڢ����м��飻��SO2����������D����Һ�лẬ��SO2��H2SO3��SO2���л�ԭ�ԣ��ʿ�ѡ�������м��顣


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����ѧƽ���ƶ�ԭ��ͬ��Ҳ����������ƽ�⡣��֪�ڰ�ˮ�д�������ƽ����
NH3+H2O![]() NH3��H2O
NH3��H2O![]()
![]() +OH-
+OH-
��1����ˮ�м���MgCl2����ʱ��ƽ���� �ƶ���OH-��Ũ�� (�������С�����䡱����ͬ)��![]() ��Ũ�� ��
��Ũ�� ��
��2����ˮ�м���Ũ������ƽ���� �ƶ�����ʱ��Һ��Ũ�ȼ�С�������� �� �� ��
��3����Ũ��ˮ�м�������NaOH������ƽ���� �ƶ�����ʱ������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ij��ѧ����С������ȡ������Na2CO3��Һ������ʵ������е�������⡣�ɹ�ѡ����Լ��У�
A������ʯ B������
C������������Һ D������ʯ��ˮ
����ͬѧ��Ƶ��Ʊ�ʵ�鷽���ķ�Ӧ���̷ֱ����£�
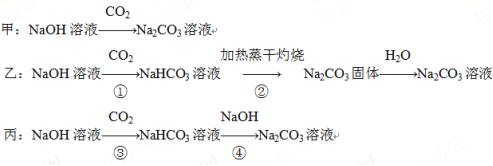
��ش�����������
(1)��ָ���ס�������������Ҫȱ�㣺
������______________________________________________________��
�ҷ�����______________________________________________________��
(2)���跴Ӧ�����õ�NaHCO3��Һ�к���Na2CO3����Ҫ��A��D�����Լ�֤��Na2CO3�Ĵ��ڣ�����ѡ����Լ���________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪһ����AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ����������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ�
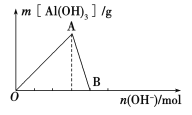
��ش��������⣺
(1)A��ʱ�Ѳμӷ�Ӧ��AlCl3��NaOH�����ʵ���֮��Ϊ________��
(2)AB����������ʾ�ķ�Ӧ�����ӷ���ʽΪ_________________________��
(3)��B�����ɵ���Һ��ͨ�������̼���ɹ۲쵽��������_______________________��
(4)����0.1 mol NH4Al(SO4)2����Һ����μ���5 mol��L1 NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����д̼�����ζ�������ݳ�������ɫ�������ٲ�������ʧ��������ͼ�л������ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵʾ��ͼ��
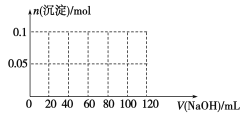
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
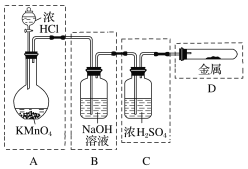
����Ŀ��(1)ʵ������ȡCl2ʱ�����в�������ȷ����________(�����)��

��װ������������ȡ����Cl2
����װ������ȥCl2�е�����HCl
����װ������ȡCl2
����װ��������Cl2
(2)ʵ������ȡ������������������˶������̡�Ũ�������Ҫ���Լ���__________��__________��__________��
(3)��֪KMnO4��Ũ�����ڳ����·�Ӧ�ܲ���Cl2��
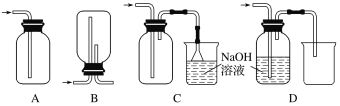
��������ͼ��ʾ��ʵ��װ�����Ʊ��������������������֤��������ķ�Ӧ��ÿ�����߿��ʾһ����Ԫװ�ã������д������________(����ĸ����ͬ)��
(4)��ʵ��������Ũ������MnO2������ȡCl2���������ʵ�顣�����ռ�Cl2��װ����ȷ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�� The general manager thought _______ of these problems before he made the final decision.
A. a good many B. a great deal
C. lots D. a plenty
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ij�о���ѧϰС��ͬѧ�����ij�п���������������ⶨ���п�����SO2�ĺ������ƶ������о�������
��.���ϱ�����SO2���н�ǿ�Ļ�ԭ�ԣ�����������������Һ������Ӧ��5SO2+2![]() +2H2O===5
+2H2O===5![]() +2Mn2++4H+��
+2Mn2++4H+��
��.���ʵ�鷽��������SO2�Ļ�ԭ��ʹSO2����֪Ũ�ȼ�����ĸ������������Һ��Ӧ��
��.ѡ������ص㣺ij��ҵ����ij����ij����С����ij��ҵ����ij��ͨ��Ŧ��
��.ѡ�����ʱ�䣺���졢���ٽ�Сʱ�����졢���ٽϴ�ʱ����ǰ�����
��.�Բⶨ�Ľ�����г�������������ص�λ������顣
(1)��С��ͬѧ������ͼ��ʾװ�ö�������������SO2�ĺ�����
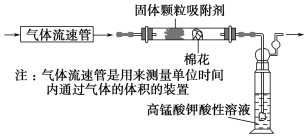
��ͨ�����ǰӦ���е�ʵ�������________________________________��
�����۲쵽ʵ�������Ϊ________________________________________ʱ��Ӧֹͣͨ������
��ʵ����������������¼��������_________________________________��
(2)��ͼ��ʾ��С��ͬѧ����ʵ���õĸ��в�ͬ����������SO2�ĺ�����
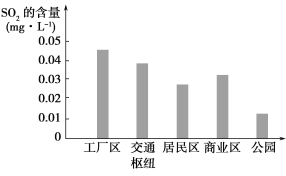
������������ͨ��ŦSO2�������Ը�������������ԭ����_____________��
�������йز������һ�����ٹ�����SO2�ŷŵĽ��飺__________________________��
(3)�±��Ǹ�С��ͬѧ��õIJ�ͬ��������µij��п�����SO2��ƽ�������������������ٽϴ�ʱSO2ƽ�������ϵ͵�ԭ��
��_____________________________________________________��
��_____________________________________________________��
������� | ƽ������(m��s1) | ������SO2��ƽ������(mg��L1) |
��ǰ | 2.0 | 0.03 |
��� | 2.2 | 0.01 |
�� | 23 | 0.015 |
�� | 0.9 | 0.03 |
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��It suddenly occurred to him___ he had left his keys in the office.
A. whether ;
B. where
C. which
D. that
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��She has already tried her best. Please don't be too______about her job.
A. special
B. responsible
C. unusual
D. particular
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com