
����Ŀ��(1)ʵ������ȡCl2ʱ�����в�������ȷ����________(�����)��

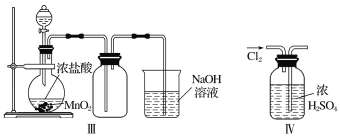
��װ������������ȡ����Cl2
����װ������ȥCl2�е�����HCl
����װ������ȡCl2
����װ��������Cl2
(2)ʵ������ȡ������������������˶������̡�Ũ�������Ҫ���Լ���__________��__________��__________��
(3)��֪KMnO4��Ũ�����ڳ����·�Ӧ�ܲ���Cl2��
��������ͼ��ʾ��ʵ��װ�����Ʊ��������������������֤��������ķ�Ӧ��ÿ�����߿��ʾһ����Ԫװ�ã������д������________(����ĸ����ͬ)��
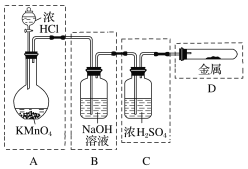
(4)��ʵ��������Ũ������MnO2������ȡCl2���������ʵ�顣�����ռ�Cl2��װ����ȷ����________��
���𰸡�(1)�٢ڢۢ�
(2)����ʳ��ˮ ŨH2SO4 NaOH��Һ
(3)BD
(4)C
��������(1)��ʵ������ȡCl2��Ũ�����MnO2��Ӧ��������ϡ�������ȥCl2�е�HCl�����ñ���ʳ��ˮ��NaHCO3��Һ��Cl2��Ӧ���ų�CO2���壬����Ӧ�������̳�������MnO2��Ũ�������ڼ��������·�Ӧ��������Cl2ʹ��ŨH2SO4ϴ��ƿ��Ӧ�������̳�����
(2)��ȡ����Cl2���豥��ʳ��ˮ��ȥHCl���壬ŨH2SO4����Cl2��NaOH��Һ��ȥ�����Cl2��
(3)B��NaOH��Һ��Cl2�ܷ�Ӧ��D���Թ�ȱ�ٳ��������ҽ�����ĩ��ͨ��NaOH��Һ�С�
(4)����ƿ��ʹ�õ�������������ֻ�ܽ�����������������ƿ��ѹǿ����һ���̶ȣ����ܽ�����ѹ����A�������ΪCl2�ȿ������ܶȴ�Cl2Ӧ�̹ܽ���B�����װ�ü����ռ�Cl2�����ܽ���β�������ҷ�������C����ȷ������Cl2��NaOH��Ӧ�����Բ����ռ���Cl2��D�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��[2016�����ѡ]�����Ƿ�չ�е�����Դ���������ð�������Ʊ��������Ӧ���������ڡ��ش��������⣺
��1����������ȣ�������Ϊȼ�ϵ��ŵ���_________(���ٴ������)����������ֱ��ȼ�յ�����ת����Զ����ȼ�ϵ�أ�д����������ȼ�ϵ�صĸ�����Ӧʽ��____________��
��2������̫����ֱ�ӷֽ�ˮ���⣬�����������������;����������ת����ʽΪ_______��
��3�����������ĸ�����Ҳ����������Դ����ⷨ��ȡ�й㷺��;��Na2FeO4��ͬʱ���������Fe+2H2O+2OH![]() FeO42+3H2��������ԭ����ͼ1��ʾ��װ��ͨ������缫���������Ϻ�ɫFeO42�����缫�����ݲ�����������������ҺŨ�ȹ��ߣ����缫����������ɫ���ʡ���֪��Na2FeO4ֻ��ǿ�����������ȶ����ױ�H2��ԭ��
FeO42+3H2��������ԭ����ͼ1��ʾ��װ��ͨ������缫���������Ϻ�ɫFeO42�����缫�����ݲ�����������������ҺŨ�ȹ��ߣ����缫����������ɫ���ʡ���֪��Na2FeO4ֻ��ǿ�����������ȶ����ױ�H2��ԭ��

�����һ��ʱ���c(OH)���͵�������_______(������������������������)��
���������У��뽫�������������弰ʱ�ų�����ԭ��Ϊ_______��
��c(Na2FeO4)���ʼc(NaOH)�ı仯��ͼ2����ѡM��N�����е�һ�㣬����c(Na2FeO4)�������ֵ��ԭ��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���Ʊ�пӡˢ��·������ϡ���ḯʴп���������ķ�Һ�ơ��ð�Һ�������ð�Һ���г�������п��������������ˮ�����Cl��Fe3+����ʵ���������á��ð�Һ����ȡZnSO4��7H2O�Ĺ�����ͼ��ʾ��
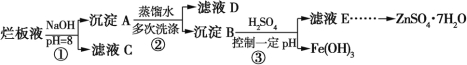
��1����ϡ���ḯʴп��ʱ��ԭ����ΪN2O���������뻹ԭ�������ʵ���֮���� ��
��2������������pH>12����Zn(OH)2�ܽ�����ƫп���ơ�Zn(OH)2�ܽ�����ӷ���ʽΪ ��
��3����ҺD�г��˺���OH���������е��������� (�����ӷ���)��
��4������ҺE��pH=4��c(Zn2+)=2 mol��L1��c(Fe3+)=2.6��109mol��L1������õ��ܶȻ��� ��
A��Ksp [Zn(OH)2] B��Ksp [Zn(OH)2]��Ksp [Fe(OH)3] C��Ksp [Fe(OH)3]
��5��������Ҫ����pH��һ����Χ��ʵ������pH��ֽ�ⶨ��ҺpH�ķ����� ��
��6����֪����Fe(OH)3(s)![]() Fe3+(aq)+3OH(aq) ��H=a kJ��mol1
Fe3+(aq)+3OH(aq) ��H=a kJ��mol1
��H2O(l)![]() H+(aq)+OH(aq) ��H=b kJ��mol1
H+(aq)+OH(aq) ��H=b kJ��mol1
�������ܶȻ�����ΪKsp���������ӻ�����ΪKw��Fe3+����ˮ�ⷴӦ��ƽ�ⳣ����K= (�ú�Ksp��Kw�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ijУ��ѧ��ȤС������ͼ��ʾ���̳�ȥAlCl3�к��е�Mg2+��K+�������Ӳ������ܼ���AlCl3����ʧ����ش��������⣺
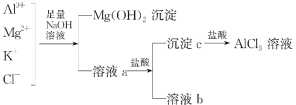
(1)д��������м�����������������Һʱ����Һ�з�����Ӧ�����ӷ���ʽ��_______________________��
(2)����������Һ�ܷ��ð�ˮ���棬Ϊʲô��___________________________��
(3)��Һa�д��ڵ�������________________������Һa�м�������ʱ��������������Ϊʲô��__________________________________��Ϊ�ˣ��Ľ�������___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾװ�ý���ʵ�飬���ش��������⣺
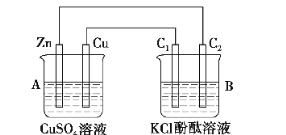
��1���ж�װ�õ����ƣ�A��Ϊ________��B��Ϊ________��
��2��п��Ϊ__________�����缫��ӦʽΪ____________________________________________��
ͭ��Ϊ__________�����缫��ӦʽΪ_____________________________________________��
ʯī��C1Ϊ__�����缫��ӦʽΪ________��ʯī��C2����������ʵ������Ϊ
________________________________________________________________________��
��3����C2������224 mL����(��״��)ʱ��п������________(����ӡ����١�)________g��CuSO4��Һ������________(����ӡ����١�)________g��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��Ϊ��̽��Cl2��SO2ͬʱͨ��H2O�з����ķ�Ӧ��ijУ��ѧ��ȤС��ͬѧ�������ͼ��ʾ��ʵ��װ�á�
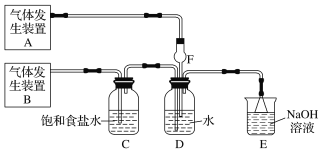
(1)�û�ѧ��ȤС���ͬѧΪ��ȡCl2��SO2���壬�ֲ���Na2SO3��70%������Ϊԭ����ȡSO2������MnO2��Ũ����(12 mol��L1)Ϊԭ����ȡCl2���ڴ�ʵ���У�F������������________������װ��BӦѡ����������װ���е�________(�����)��

(2)Dװ������Ҫ��Ӧ�����ӷ���ʽΪ_________________________________��
(3)Ϊ��֤ͨ��Dװ���е�������Cl2��������SO2��������ȤС���ͬѧ���������Լ���
���Ȼ�����Һ ���Ȼ�������Һ �����軯����Һ �����Ը��������Һ
��Cl2������ȡ����D����Һ�μ���ʢ��________(ѡ��һ�����)�Լ����Թ��ڣ��ټ���________(ѡ��һ�����)�Լ���������������______________________________________��
��SO2������ȡ����D����Һ�μ���ʢ��________(ѡ��һ�����)�Լ����Թ��ڣ�������������_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�� However hard I tried, I just couldn��t ______them of the truth of my story.
A. remind B. convince C. rid D. inform
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��(2017�����)Mr. and Mrs. Brown would like to see their daughter ___________, get married, and have kids.
A. settle down B. keep off C. get up D. cut in
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����ͼΪ������ɽ���������ﷴӦ������ij�ֵ����ʵ�����ͼ��������ش�
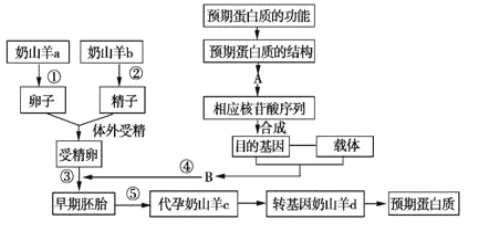
��1��ͼ��A��B�ֱ��ʾ�� �� ��
��2���������ü��ض���ɽ��a����________�������������ж���ɽ��b�����ľ����������ܾ�ǰ����________�����л��ܴ������Բɼ�����ĸϸ��Ҫ������������ ________�ڣ���������ܵľ����ܾ���������������________����Ŀ�Ļ������ܾ����ڡ��ڽ�������̥��ֲ�������ɽ���ӹ���֮ǰ������������̥ϸ���Ƿ��Ѿ�����Ŀ�Ļ����˲���_______________������
��3�������ʹ����У�Ҫ�Ե����ʽṹ������Ƹ��죬����ͨ���������λ����ϳ�����ɣ�����ֱ�Ӹ��쵰���ʣ�ԭ����__________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com