
����Ŀ��ij���Ӿ��徧���ṹ��ͼ��ʾ��(![]() )Xλ��������Ķ��㣬(��)Yλ������������ģ��Է�����
)Xλ��������Ķ��㣬(��)Yλ������������ģ��Է�����
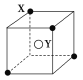
(1)������ÿ��Yͬʱ������________��X��ÿ��Xͬʱ������________��Y���þ���Ļ�ѧʽΪ________��
(2)��������ÿ��X��Χ������ӽ��Ҿ�����ȵ�X����________����
(3)�����о��������2��X��һ��Y�γɵļнǡ�XYX��________(��ǵĶ���)��
(4)������������ⳤΪa cm�������ܶ�Ϊ�� g��cm��3��NAΪ�����ӵ�������������ӻ������Ħ������Ϊ________��
���𰸡�(1)4 8 XY2(��Y2X) (2)12
(3)109.5��(4)2a3��NA g��mol��1
��������(1)�ɾ�����ֱ�ӿ���ÿ��Y��Χ������4��X��ÿ��X��8���������ã���X��Χ��8���Ⱦ����Y��������X��Y����Ŀ��Ϊ![]() ��1��1��2���ʻ�ѧʽΪXY2��Y2X��
��1��1��2���ʻ�ѧʽΪXY2��Y2X��
(2)��ij��XΪ���ģ�����8������X�ľ������ɷ���������X�Ⱦ����������X����3�㣬ÿ��4������12����(3)�ĸ�XΧ��һ���������壬Yλ�����ģ����Ƽ���ķ��ӽṹ���ʡ�XYX��109.5�㡣(4)ÿĦ�����൱�ں���0.5 mol XY2(��Y2X)����Ħ�������ɱ�ʾΪM��![]() g��mol��1��2a3��NAg��mol��1��
g��mol��1��2a3��NAg��mol��1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����ʯ�е�CaC2��H2O��Ӧ��������C2H2��CaC2��2H2O��C2H2����Ca(OH)2��ͨ����ˮ��������Ӧ�����������������Ӷ��ɲⶨ��ʯ��̼���Ƶĺ�����
(1)��ʹ�����������͵�����װʵ��װ�ã�������ʾ��
������ ���� |
|
|
|
������ ���� |
|
|
|
ÿ����Ƥ�������������� | |||
������������������ͨ��ʱ�����������͵��ܴ�����ֱ�����ӵ�˳����(�������뵼�ܵ����)��
________��________��________��________��________��________��
(2)�������Ӻú���ʵ��ʱ�������в���(ÿ�����ֻ����һ��)��
�ٳ�ȡһ������ʯ����������3�У�������Ƥ����
�ڼ��װ�õ������ԡ�
��������6��5��ע������ˮ��
�ܴ�����3�ָ�������ʱ����ȡ����4��ˮ�����(����2��ˮ���Բ���)��
��������������6�Ļ�����ʹˮ��ε���������������Ϊֹ���رջ�����
��ȷ�IJ���˳����(�ò���������)________��
(3)��ʵ����������������ŵ���ζ���Ҳⶨ���ƫ��������Ϊ��ʯ�к���________���ʡ�
(4)��ʵ��ʱ��ȡ��ʯΪ1.60 g����������ˮ�����������ɱ�״������Ȳ�����Ϊ448 mL����˵�ʯ��̼���Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ������Ԫ�ص�ԭ�ӵ��Ӳ�ṹ���£�
A��1s22s22p63s23p63d54s1��B.1s22s22p63s2��C.1s22s22p6��D.1s22s22p63s23p��E.[Ar]4s1��
�Իش�
(1)�ĸ�ʽ���Ǵ����________����Υ����________��
(2)�ĸ�ʽ�ӱ�ʾϡ������ԭ��________��
(3)A��Ԫ�ط�����________��д����۵����Ų��Ĺ����ʾʽ________��
(4)B��E�Ľ�����ǿ����ϵΪ________(��Ԫ�ط��ű�ʾ)������˵����һ��ʵ��������(д��2��)________________________ _��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����֪��̼Ԫ��ͬ�����XԪ��λ�����ڱ��еĵ�1�������ڣ�������Ԫ��Yԭ�ӵ��������������ڲ����������3���������γɻ�����ķ���ʽ��XY4���Իش�
(1)XԪ�ص�ԭ�ӻ�̬ʱ�����Ų�ʽΪ__________��YԪ��ԭ���������ӵĵ����Ų�ͼΪ__________��
(2)��X��Y��Ԫ�ص縺�Էֱ�Ϊ2.1��2.85����XY4��X��Y֮��Ļ�ѧ��Ϊ__________(����ۼ��������Ӽ���)��
(3)�û���������幹��Ϊ__________������ԭ�ӵ��ӻ�����Ϊ__________������Ϊ__________(����Է��ӡ��Ǽ��Է��ӡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����֪As2O3��Zn���Է������·�Ӧ��As2O3+6Zn+6H2SO4![]() 2AsH3��+6ZnSO4+3H2O��
2AsH3��+6ZnSO4+3H2O��
(1)����˫���ŷ��������ת�Ƶķ������Ŀ____________________________________��
(2)As2O3��������Ӧ����ʾ������������____________(�����)��
A�������� B����ԭ�� C������ D������
(3)�÷�Ӧ������������__________����ԭ������________��
(4)������0.2 mol AsH3����ת�Ƶĵ�����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��(1)���ݷ�Ӧ8NH3+3Cl2![]() 6NH4Cl+N2���ش��������⣺
6NH4Cl+N2���ش��������⣺
���÷�Ӧ���������� ������������ ��
���÷�Ӧ�б������������뱻��ԭ���������ʵ���֮��Ϊ ��
(2)ijһ��Ӧ��ϵ�д�������6�����ʣ�NO��FeSO4��Fe(NO3)3��HNO3��Fe2(SO4)3��H2O����֪��������ת����ϵ��HNO3��NO����������и��⣺
���÷�Ӧ���������� ����ԭ���� ��
���÷�Ӧ��1 mol������ (��õ�����ʧȥ��) mol���ӡ�
������0.1 mol HNO3����ԭ����ʱ���ɱ�״����NO������� L��
����Ѹ�������������ո�����ƽ��
��______+��______![]() ��______+��______+��______+��
��______+��______+��______+��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����һ���Լ���ȥ���и������е�����(������Ϊ����)����д�����ӷ���ʽ��
(1)BaCl2(HCl)���Լ�________�����ӷ���ʽΪ_________________________��
(2)O2(CO2)���Լ�________�����ӷ���ʽΪ__________________________��
(3)![]() (
(![]() )���Լ�________�����ӷ���ʽΪ_______________________��
)���Լ�________�����ӷ���ʽΪ_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽS(l)+O2(g)![]() SO2(g) ��H1=293.23 kJ��mol1����������˵����ȷ����
SO2(g) ��H1=293.23 kJ��mol1����������˵����ȷ����
A����ӦS(s)+O2(g)![]() SO2(g)����ЧӦС�ڦ�H1
SO2(g)����ЧӦС�ڦ�H1
B����ӦS(g)+O2(g)![]() SO2(g)����ЧӦ���ڦ�H1
SO2(g)����ЧӦ���ڦ�H1
C��1 mol SO2(g)������С��1 mol S(l)��1 mol O2(g)������֮��
D��1 mol SO2 (g)����������1 mol S(l)��1 mol O2(g)������֮��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����֪AΪ�����Ľ������ʣ�������ͼ��ʾ�Ĺ�ϵ���ش��������⡣
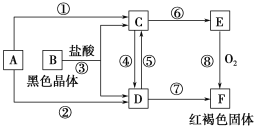
(1)ȷ��A��B��C��D��E��F�Ļ�ѧʽ�� AΪ________��BΪ________��CΪ________��DΪ________��EΪ________ ��FΪ________��
(2)д����Ӧ���Ļ�ѧ����ʽ�ͷ�Ӧ�����������ӷ���ʽ��
��____________________________________________________________��
��_____________________________________________________________��
��_____________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com