
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�~���ڱ��е�λ�ã��û�ѧ����ش��������⣺
| ���� | ��A | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | | �� | �� | |
���������������1����Ԫ��Ϊ�������ڵ�������Ԫ������ԭ������Ϊ13�������ӽṹʾ��ͼΪ
��2��Ԫ�آ�ΪSԪ�أ�����������������ȣ�����������32����ͬλ�ط�����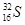 (��32S)��
(��32S)��
��3���ܡ��ݡ��ߵ����ӷֱ���O2-��Na+��S2-�����ݰ뾶�Ĵ�С��Ҫ�ɵ��Ӳ������˵�������������Ӳ���Խ��뾶Խ�˵����Խ��뾶ԽС������3�����ӵİ뾶��С˳��ΪS2->O2-> Na+��
��4����Ϊ̼Ԫ�أ�����������Ӧˮ����Ϊ̼�ᣬ�������ᣬ����뷽��ʽΪ��H2CO3? HCO3- + H+ HCO3- ? CO32- + H+��
��5���ܡ�������Ԫ�طֱ�Ϊ������Ԫ�أ�ԭ�Ӱ�1:1��ɵĻ�����ΪNa2O2,�����ʽΪ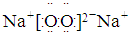
��6���ɱ�������Ԫ���γɵ�5��10���ӷ���Ӧ��CH4���õ���ڼ��������µĸ�����ӦʽΪ��CH4-8e-+10OH-= CO32-+7H2O
��7��A��B������18�����ӣ�A��һ��6ԭ�ӷ��ӣ��������������ȼ�ϣ���AΪN2H4, B��һ�ֳ���ǿ������,��BΪH2O2, �����������ݿɵ�A��B��Ӧ���Ȼ�ѧ����ʽ��N2H4(l)+2H2O2(l) = 4H2O(l)+N2(g) ��H="-1676" kJ/mol
���㣺����Ԫ�����ڱ���ԭ�ӽṹ����ѧ�������롢��ѧ��Ӧ��������ȼ�ϵ�ص����֪ʶ���ۺ�Ӧ�á�ͬʱ����ѧ���������������������


 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ڹ���Ԫ��Fe��Ti����C��H��N��O�γɶ��ֻ����
��1����H��C��N��0����Ԫ�صĵ縺����С�����˳��Ϊ_____________________��
��������������ȷ����_____________��������ĸ��
A����ΪHCHO��ˮ���Ӽ����γ����������HCHO������ˮ
B��HCHO��CO2�����е�����ԭ�Ӿ����� �ӻ�
�ӻ�
C��C6H6�������6�� ����1����
����1���� ����C2H2�ǷǼ��Է���
����C2H2�ǷǼ��Է���
D��CO2������۵㡢�е㶼�ȶ������辧��ĵ�
��2��Feԭ�ӻ�������Χ�н϶���������Ŀչ������һЩ���ӻ������γ�����
����Feԭ�ӻ������γ������ķ��ӻ�����Ӧ�߱��Ľṹ������__________��
���������������[Fe(CN)6] �����______________________________________��
�����______________________________________��
A�����ۼ� B���Ǽ��Լ� C����λ�� D�� �� E��
�� E�� ��
��
д��һ����CN����Ϊ�ȵ��������ӵķ���ʽ_____________________��
��3�� SO3���ӵ����幹��Ϊ_____________��SeO32�������幹��Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̼���仯����Ӧ�ù㷺��
I.��ҵ������CO��ˮ������Ӧ����������������ƽ��:CO(g)+H2O(g) 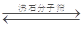 CO2(g)+H2(g)
CO2(g)+H2(g)
��1����ʯ����ɸ�к��й�Ԫ�أ���д����ԭ�ӽṹʾ��ͼ__________��
��2����1L�����ܱ�������ע��CO��H2o(g),830��ʱ��ò����������±�������¶��·�Ӧ��ƽ�ⳣ
��K=______________��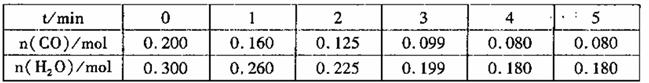
��3����ͬ�����£���1L�����ܱ������У�ͬʱע��1mol CO��1mol H2O(g),2molCO2��2mo1 H2����ʱv(�� ) __________v(��)(���������������)
II.��֪CO(g)+1/2 O2 (g)��CO2 (g) ��H��һ141 kJ��mol-1
2H2(g)+ O2(g)��2H2O(g) ��H��һ484 kJ��mol-1
CH3OH��1��+3/2O2 (g)��CO2(g)+2H2O(g) ��Hl��һ726 kJ��mol-1
��4������CO��H2�����Ƶ�Һ̬�״����Ȼ�ѧ����ʽΪ___________��
III.һ����������ȼ�ϵ�ع���ԭ������ͼ��ʾ
��5��д���缫A�ĵ缫��Ӧʽ_____________��
��6����������ص�ⱥ��ʳ��ˮ��������0.2mo1 Cl2����������ͨ��O2�����Ϊ_____L(��״��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�ֶ�����Ԫ�ص�ԭ��������E��D��B��A��C��˳����������A��Cͬ���ڣ�B��Cͬ���壻A��B���γ����ӻ�����A2B��A2B���������ӵĵ�������ͬ���ҵ�������Ϊ30��D��E���γ�4��10���ӵķ��ӡ��Իش��������⣺
��1���õ���ʽ��ʾ���ӻ�����A2B���γɹ��̣�__________________________________________________________________________��
��2��д���������ʵĵ���ʽ��
DԪ���γɵĵ���__________��B��E�γɵĻ�����E2B__________��A��B��E�γɵĻ�����__________��D��E�γɵĻ�����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ�������������Ϣ���ɡ��±����������ֶ����ڵ�ԭ�Ӱ뾶����Ҫ���ϼۣ���֪���ԭ�Ӱ뾶Ϊ0.089 nm����
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.16 | 0.143 | 0.102 | 0.099 | 0.074 |
| ��Ҫ���ϼ� | ��2 | ��3 | ��6����2 | ��1 | ��2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ԫ�����ڱ���ǰ7���ڵ�Ԫ���������±���ʾ��
| ������ | һ | �� | �� | �� | �� | �� | �� |
| Ԫ������ | 2 | 8 | 8 | 18 | 18 | 32 | 32 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ԫ��A��B��C��D�����ڱ��е�λ����ͼ,E2+��D�ļ�����������ͬ�ĵ��Ӳ�ṹ,�ش���������: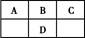
(1)��A��B��C��D����Ԫ�ص�ԭ������֮��Ϊm,��m��������
A.һ��Ϊ����
B.һ��Ϊż��
C.����Ϊ����,Ҳ����Ϊż��
(2)DԪ��ԭ�ӵĴ������������������������֮��,��:
��д��A�γɵļ����ӵĽṹʾ��ͼ��������,Ԫ��Dλ��Ԫ�����ڱ��ĵ����������塣
��AԪ�ص�һ���⻯���������6��ԭ��,��ṹ��ʽΪ������������������ѹ298 Kʱ0.2 mol����̬�⻯����O2����ȫȼ��,������̬A���ʺ�ˮ,�ų�����106.8 kJ,����̬�⻯��ȼ�յ��Ȼ�ѧ����ʽΪ�� ����
��д��C������B������⻯�ﷴӦ�Ļ�ѧ����ʽ:
��ʵ��֤ʵAC3��ˮ�ᷢ����Ӧ����HNO2��HF,��AC3��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͼ������ͼ���dz��õĿ�ѧ�о�������
��.ͼ(A)�Ƕ�����ij����Ԫ��X�ĵ�������ʾ�������XԪ��λ�����ڱ��ĵ����������塣 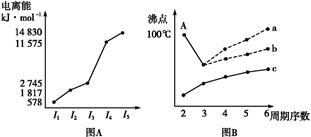
ͼB���о�����Ԫ�ص��⻯��ķе�仯���ɵ�ͼ��,����c���Ա����������������Ԫ���⻯��ķе�ı仯���ɡ�
��.�±���Ԫ�����ڱ���һ����,�������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
�Իش���������:
(1)��д��Ԫ��o����Χ�����Ų�ʽ:������������������
(2)��jԭ�Ӹ�cԭ����1��1������϶��γɵľ���,�����뾧��j��ͬ����������۵���ߵ���
��������(�ѧʽ),�Դӽṹ�Ƕȼ��Խ���:��
(3)i���ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ,�侧����������ͼ����ʾ,ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ��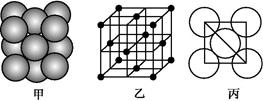
��ش�:������iԭ�ӵ���λ��Ϊ��������,һ��������iԭ�ӵ���ĿΪ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��M��Q�����ֶ���������Ԫ�أ�ԭ�������������� X��Y���γ�����ܼ���X��Y��Z��Ϧ�������֮��Ϊ8��Y��M�γɵ���̬�������ڱ�״���µ��ܶ�Ϊ2.86g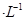 ����ش��������⣺
����ش��������⣺
��1��Y��Z��M����Ԫ��ԭ�Ӱ뾶��С�����˳��Ϊ��дԪ�ط��ţ�________________��
��2��Q�����ڱ��е�λ����____________��
��3��X��Y��Q����Ԫ�ء��γɵ���ԭ�ӷ��ӽṹʽΪ______________��M��QԪ���γɵ�����������Ӧ��ˮ�����н�ǿ�����ǣ�д��ѧʽ��_____________��
��4��Y��Z�γɵĻ�����A�Ⱥ����Ӽ��ֺ����ۼ�,A�ĵ���ʽΪ______________________��
��A����ʢ�з�̪��Һ���Թ��й۲쵽������Ϊ_________________________________��������Ӧ�Ļ�ѧ����ʽΪ_________________��
��5����������װ�ÿɽ�������MY2ת��Ϊ��Ҫ����ԭ��H2MY4���缫a�Ĵ������淢���ĵ缫��Ӧ����ʽΪ__________________________________��������ͨ��MY2��O2�����ʵ�����ѱ�ֵΪ________________��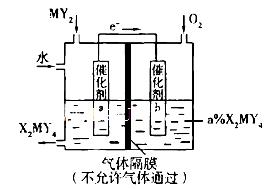
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com